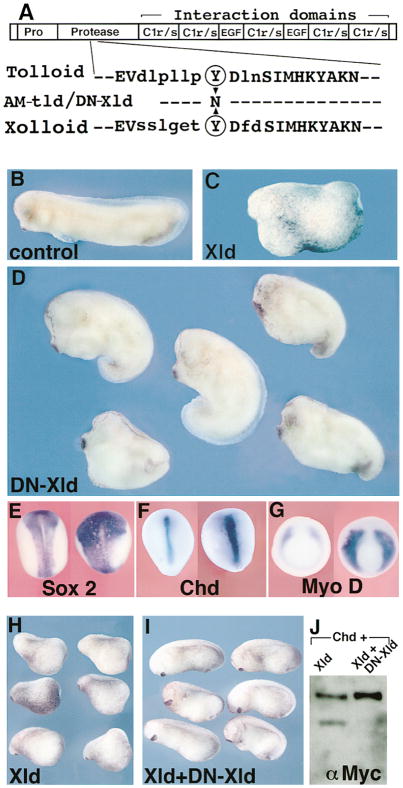
Figure 6. In Vivo Role of Xolloid: Expansion of Organizer Activity in Embryos Injected with Dominant-Negative Xolloid.
(A) Top, schematic structure of Xolloid: EGF and C1r/s are protein–protein interaction domains. Bottom, sequence of the protease domain of Drosophila Tolloid and of Xenopus Xolloid surrounding a conserved tyrosine (circled, position 272 in Tolloid and 296 in Xolloid) mutated in DN-Xld.
(B) Control stage 29 embryo.
(C) Xld gain-of-function: ventralized embryo injected with 200 pg of XLD mRNA in each blastomere at the 4-cell stage.
(D) Dorsalization in embryos similarly injected with 300 pg of DN-XLD mRNA (76%, n = 127). Compare with sibling embryo in (B); the head and cement gland are enlarged, reflecting dorsalization of the embryos.
(E–G) In situ hybridization for the indicated markers in control (left) or DN-XLD mRNA–injected (right) embryos. The neural domain marked by Sox-2 (E) and the dorsal mesoderm domains marked by Chd (F) or MyoD (G) are expanded and increased in intensity in DN-Xld-injected embryos (n = 24, 10, and 15, respectively), indicating increased organizer activity. The injected embryo in (E) is shortened due to dorsalization.
(H) Stage 20 embryos injected in the two dorsal blastomeres at the 8-cell stage with 200 pg of Xolloid mRNA.
(I) Normal development is restored by coexpression of XLD mRNA (200 pg) and DN-XLD mRNA (800 pg). This indicates that DN-Xld can antagonize wild-type Xld.
(J) Western blot showing that DN-Xld protein produced by cotransfection inhibits the enzymatic activity of Xolloid on Chordin substrate.