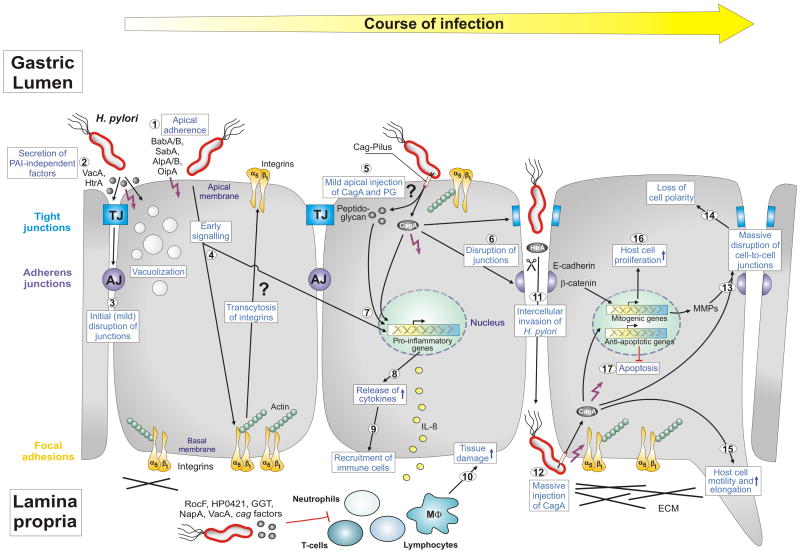
Figure 1.
Model of Helicobacter pylori-induced epithelial-barrier disruption and pathogenesis. The interplay between polarized gastric epithelial cells and a variety of bacterial pathogenicity factors modulates multiple host responses during the course of infection, as indicated. H. pylori expresses several adhesins such as BabA, BabB, SabA, AlpA and AlpB as well as OipA which mediate apical binding of the bacteria (1). Attached H. pylori or those in the mucus secrete virulence factors into the medium (HtrA protease, VacA cytotoxin and others) (2), which could trigger mild opening of tight junctions (TJs) and adherens junctions (AJs) at early time points of infection (3). While internalized VacA causes cellular vacuolization, a hallmark of the ulceration process, HtrA cleaves the junctional protein E-cadherin [8]. Another intriguing possibility of junction disruption could be the transcytosis of basal integrins to the apical surface by early, but unknown, cagPAI-independent signaling (4). Apical exposure of some integrin molecules such as integrin α5β1 could stimulate the T4SS pilus to inject CagA and peptidoglycan into cells (5). Injected CagA can then be targeted to TJs and AJs followed by further disruption of these junctions (6). Injected CagA and peptidoglycan (5) in addition to OipA (1) can trigger nuclear factor-kB (NF-κB) activation (7) and the release of proinflammatory cytokines such as IL-8 (8). These cytokines can alter the secretion of mucus and induce changes in gastric-acid secretion and homeostasis. They also attract immune cells to infiltrate from the blood stream into the gastric mucosa (9), where they cause substantial tissue damage at the site of infection (10). H. pylori also express numerous factors to suppress immune cell functions as indicated. The result of the above described processes is local epithelial disruption enabling some H. pylori of entering the intercellular space between adjacent cells and reaching the basal membranes (11). In this manner, the bacteria could access integrins and inject CagA (12). Injected CagA can then induce the massive disruption of cell junctions (13) and loss of cell polarity (14). The induction of metalloproteases (MMPs) might enhance this effect in addition to HtrA. Finally, CagA can induce multiple pathways to trigger host-cell motility and elongation (15) and the onset of mitogenic genes and cell proliferation (16), and it can inhibit apoptosis (17). The interplay of each of these pathways could result in substantial deregulation and oncogenic transformation of gastric epithelial cells in vivo and, thus, are important for H. pylori pathogenesis. Specific steps labeled with question marks are untested or speculative aspects of the model. Other specific abbreviations used: α5β1 (chains of the integrin receptor), cag (cytotoxin-associated genes), ECM (extracellular matrix), IL-8 (interleukin 8), HP0421 (cholesterol-alpha-glucosyltransferase), MΦ (macrophage), NapA (neutrophil-activating protein A), PAI (pathogenicity island), PG (peptidoglycan), RocF (arginase enzyme). For more details see text and glossary in the Supplementary Material (Doc. S1). This model was updated from Wessler and Backert [15] with kind permission from Blackwell Publishing.