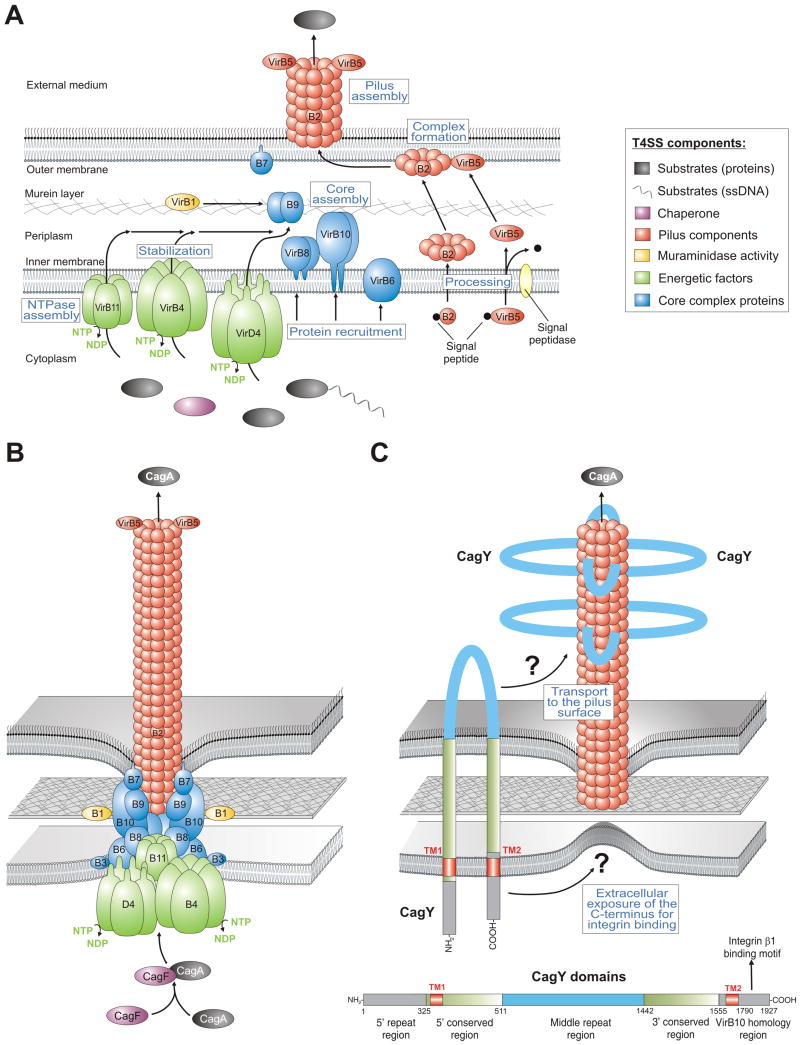
Figure 2.
Models for the assembly and assembled structure of T4SSs in Agrobacterium tumefaciens and Helicobacter pylori. (A) Proposed assembly of the prototypical Agrobacterium VirB/VirD4 T4SS machinery is shown. The T4SS is a multi-component protein complex spanning the inner and outer membranes of Agrobacterium and other Gram-negative bacteria. Current knowledge of T4SS assembly, cellular localization of its components is shown in a simplified manner. The coupling protein VirD4 and structural components (VirB1-VirB11) are typically required for secretion and are depicted according to their proposed functions. A model for T-pilus assembly in Agrobacterium showing the proposed VirB4-VirB8-VirB5-VirB2 interaction sequence leading to the formation of VirB2-VirB5 complexes followed by T-pilus assembly. The assembled T4SS then triggers the secretion of substrates from the bacterial cytoplasm directly into the cytoplasm of infected host cells or into the extracellular milieu. (B) Model-1 for the assembled T4SS machinery in H. pylori assuming that all VirB1-11 proteins are encoded by the cagPAI and assemble in a similar fashion as proposed for A. tumefaciens [6]. The reported substrates for this T4SS are CagA and peptidoglycan. (C) Model-2 proposes that the T4SS requires basically the same VirB proteins as in panel B with one major difference. The pilus surface is proposed to be covered with CagY molecules. In contrast to VirB10 in many T4SSs, H. pylori VirB10 (CagY) is a very large protein (ca. 250 kDa, domain structure and amino acid positions shown for CagY of strain 26695, accession number NP_207323.1) carrying two transmembrane domains (TM1 and TM2) to form a hairpin-loop structure in the membranes as depicted [10]. Immunogold labelling against the loop region in CagY indicated that this part of the protein is exposed to the extracellular space and is transported to the pilus surface by a yet unknown mechanism [10]. However, recent data demonstrated that the very carboxy-terminus of CagY can bind to the host receptor integrin β1 [13]. How the latter domain can be exposed to the extracellular space in order to make contact with integrin β1 is not yet clear and must be clarified in future studies.