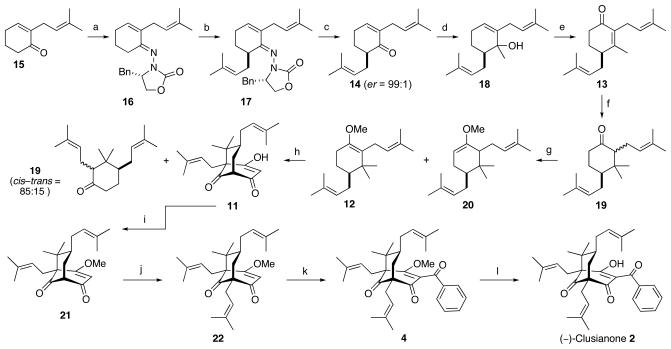
Scheme 3.
Synthesis of (−)-Clusianone (2). a) 9, p-TsOH·H2O, CH2Cl2, reflux, 95%; b) LDA, THF, −78 °C, then prenyl bromide, to 0 °C, 90%; c) p-TsOH·H2O, acetone, H2O, 80%; d) MeMgBr, Et2O, −78 °C to rt, 88%; e) PCC, 3 Å MS, CH2Cl2, 67%; f) MeMgBr, CuBr·SMe2, TMSCl, HMPA, then 10% HCl, 88%; g) t-BuOK, DMSO, then Me2SO4, 63%; h) malonyl dichloride, Et2O, −20 °C, then KOH, BnEt3NCl, H2O, 35%; i) (MeO)3CH, p-TsOH·H2O, MeOH, 50 °C, 60%; j) LDA, THF, −78 °C, then prenyl bromide, 90%; k) LTMP, THF, −78 °C, then BzCl, 65%; l) LiOH, dioxane, H2O, 90 °C, 79%.