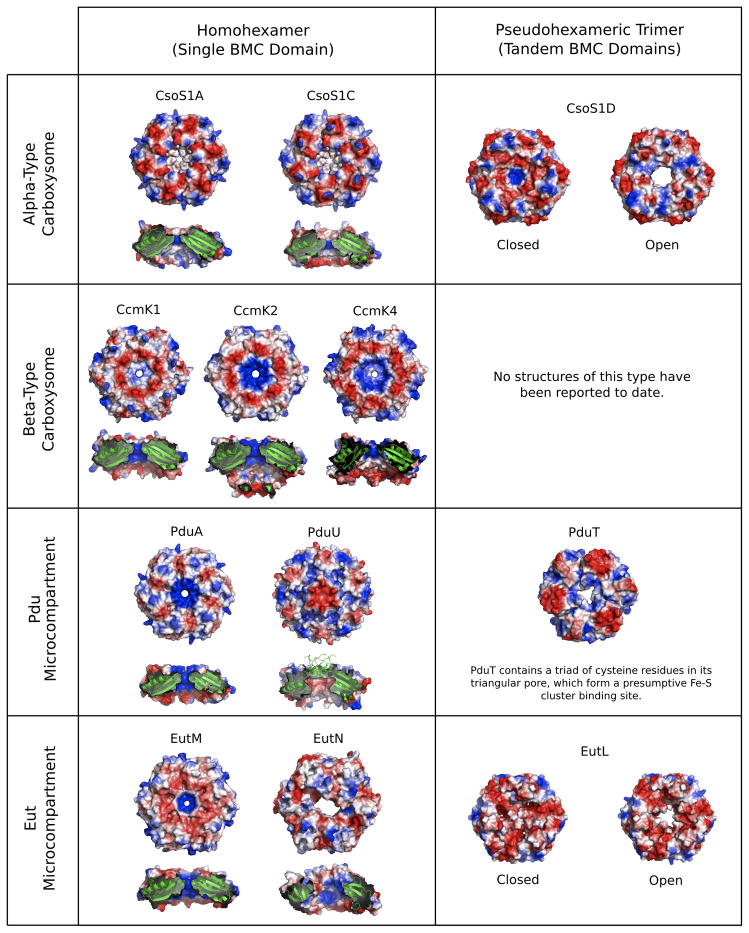
Figure 3.
BMC pore variations and transitions. Diagrams illustrate the structural diversity of several different pores in BMC shell proteins from experimentally characterized microcompartments (carboxysomes, Pdu, and Eut). Varied shell proteins exhibit differences in size, shape and electrostatic properties (blue=positive, red=negative). Some shell proteins that have tandem BMC domains are able to alter their porosity through conformational changes that open and close the pore. In addition to the presence of gated pores, other interesting pore features include: a beta-barrel that plugs an otherwise wide and negatively charged pore in PduU; a 4Fe-4S cluster binding site at the center of the PduT pore, and a distorted pore at the center of EutN, which is not made up of BMC proteins, but is instead a hexamer of CcmL/CsoS4-type protein subunits. The diverse properties illustrated are responsible for the ability of BMC shells to transport a wide range of substrates, products, and cofactors, while simultaneously limiting the efflux of metabolic intermediates. In each panel, the shell proteins are oriented so that the top surface represents the side predicted to represent the outside of the microcompartment (i.e. the side facing the cytosol). Known shell protein structures not illustrated here include CcmL, CsoS4A, EutS and EtuB.