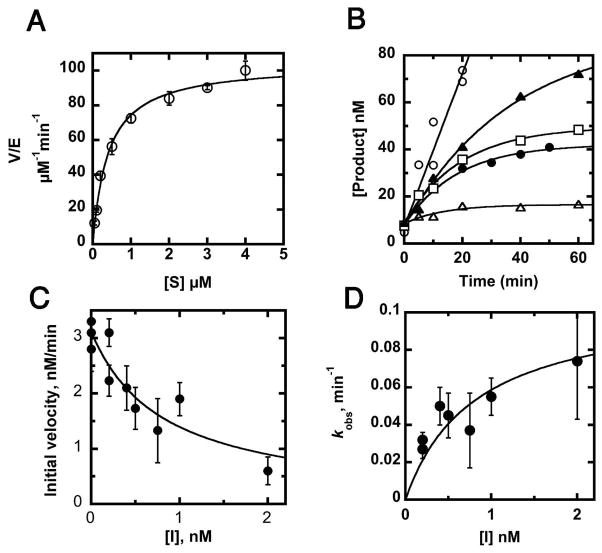
Figure 2.
Steady-state kinetics and inhibition of YeLpxC. (A) Steady-state turnover. Deacetylation of 14C-UDP-myristoyl-N-acetylglucosmine catalyzed by Zn2+-YeLpxC was assayed in 20 mM Bis-Tris (pH 7.5), 2 mM TCEP, 1 mg/mL BSA at 30° C as described in Materials and Methods. (B) Time-dependent inhibition by CHIR-090. The inhibitor CHIR-090 (0.0 nM (open circles), 0.2 nM (closed triangles), 0.4 nM (open squares), 1 nM (closed circles), and 2 nM (open triangles)) was incubated with substrate before addition of 0.1 nM Zn2+-YeLpxC and measurement of product formation. Equation 1 was fit to the progress curves (R = 0.96–0.99). (C) Measurement of inhibition constant (Ki). The value of Ki was obtained from a fit of equation 2 to the dependence of the initial velocities (obtained from the progress curves) on the concentration of CHIR-090 (R = 0.92). (D) Time-dependence of inhibition. The value of the maximal rate constant for time-dependent inhibition was calculated from a fit of equation 3 to the concentration dependence of the observed rate constants (kobs).