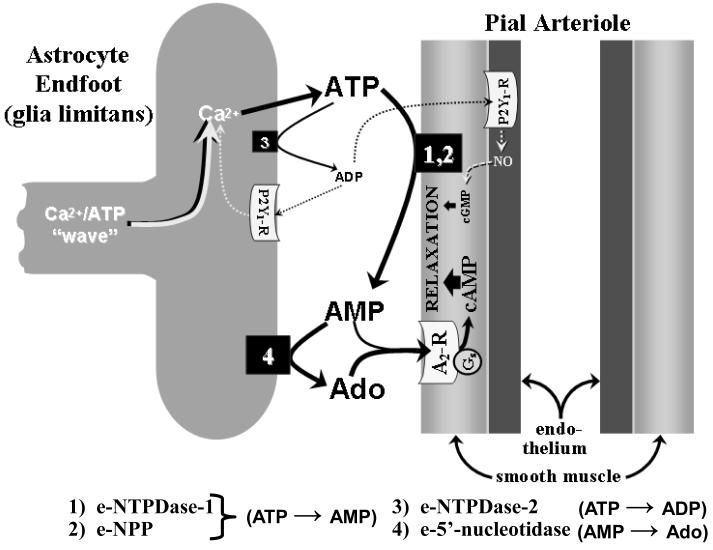
FIGURE 2.
Postulated events thought to occur following the arrival of a Ca2+/ATP “wave” at the glia limitans and the subsequent Ca2+-dependent ATP release from the glia limitans. Preliminary findings indicated that, when ATP is topically applied to the cortical surface (mimicking SNS-associated increased ATP release from the glia limitans [see fig. 1]), the ensuing dilation of pial arterioles is mediated by products of ATP hydrolysis, the first step being a rapid ATP conversion to AMP, mediated through the actions of ecto-nucleoside triphosphate dihosphohydrolase-1 (e-NTPDase-1 (1) and ecto-pyrophosphatase/phosphodiesterase (e-NPP) (2), which are found on the surfaces of vascular cells and, perhaps, astrocytes as well [72,73]. Most of the AMP is converted to adenosine (Ado) via ecto-5′-nucleotidase (4), mainly found on astrocytes [73]. Pial arteriolar dilation arises from the cAMP generated following activation of vascular smooth muscle Gs-linked A2 receptors (A2-R). The principal A2-R ligands are Ado and (to a lesser degree) AMP. If extracellular ATP elevations are sufficiently high, modest generation of ADP may occur, allowing some ATP→ADP (i.e., ecto-ATPase) activity to be manifested [74], for example, via astrocytic e-NTPDase-2 (3). ADP is known to dilate pial arterioles through activation of purinergic P2Y1 receptors found both on the glia limitans and on vascular endothelium [75], with the latter representing a minor pathway linked to NO and cGMP generation.