Figure 2.
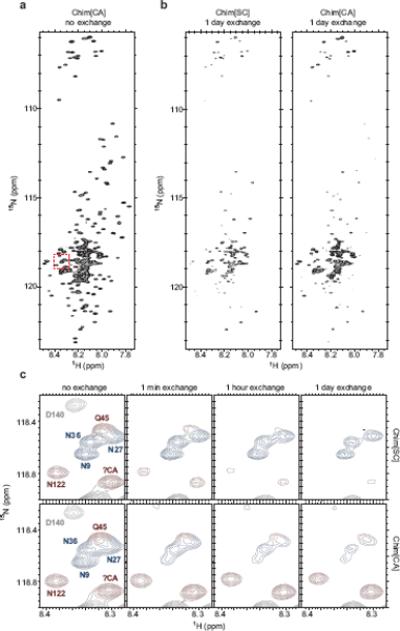
Hydrogen/deuterium exchange of Chimera fibers
Hydrogen/deuterium exchange and NMR on 15N-labeled Chim[SC] and Chim[CA] fibers was performed as previously described23 with the following modification. Rather than pelleting fibers by ultracentrifugation and resuspending fibers into D2O-containing buffer to start the exchange, freshly prepared 15N-Chimera fibers were first concentrated using an Amicon Ultra-15 centrifugal filter unit with Ultracel-30 membrane (Millipore), then diluted 12.5-fold into the equivalent buffer in D2O at pH 7.0 to start the exchange. Time points were taken at 0 minutes, 1 minute, 1 hour, and 1 day of exchange. Exchange was quenched by adjusting pH to 2.5 with DCl. (a) 15N-HSQC spectrum for Chim[CA], no exchange. Chim[SC] spectrum appears very similar. Red dashed box indicates residues shown in (c). (b) Spectra for Chim[SC] and Chim[CA], 1 day exchange. After 1 day of exchange, the same spectra for Chim[SC] and Chim[CA] qualitatively reveal large differences from the no exchange spectra and from each other. (c) A subset of residues (indicated by red box in (a)) from Chim[SC] and Chim[CA] after no exchange and 1 minute, 1 hour, and 1 day of exchange. Peaks are colored according to assignments: blue, Ch1–40 (SC-derived); red, Ch41–135 (CA-derived); gray, Ch136–253 (middle domain). Because most residues at the boundaries of the SC and CA segments of Chimera were assigned, a number of unassigned peaks (including the peak denoted as ?CA) could be identified as originating from CA residues due to the lack of a corresponding peak in SC spectra (see Supplementary methods for details on peak assignments).
