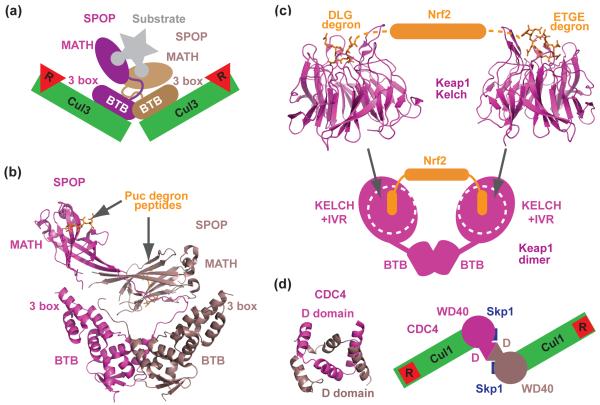
Figure 4.
Dimerization of CRL substrate receptors. (a) A schematic model showing that the asymmetric SPOP dimer recruits two copies of Cul3-Rbx1 and can bind a single substrate polypeptide by recognizing two degrons via two MATH domains [41••]. Degrons are represented by the rounded ends of substrate. (b) Crystal structure of the asymmetric SPOP dimer [41••]. Each MATH domain in SPOP is in complex with a Puc degron peptide. The BTB domains of SPOP mediate dimer formation, while the 3 box domains promote Cul3 binding. (c) Crystal structures of Keap1 Kelch β-propeller in complexes with two distinct degrons of Nrf2 [32,33,54]. A schematic drawing of the full-length Keap1 dimer is shown at the bottom based on the two-site recognition model and EM reconstruction results. Each globular lobe of the Keap1 dimer contains a Kelch β-propeller and the IVR region. (d) Crystal structure of the dimeric Cdc4 D-domain and a top view of a schematic model of a dimeric SCFCdc4 proposed in [62]. The WD40-repeats domains of the Cdc4 dimer are represented by two circles.