Abstract
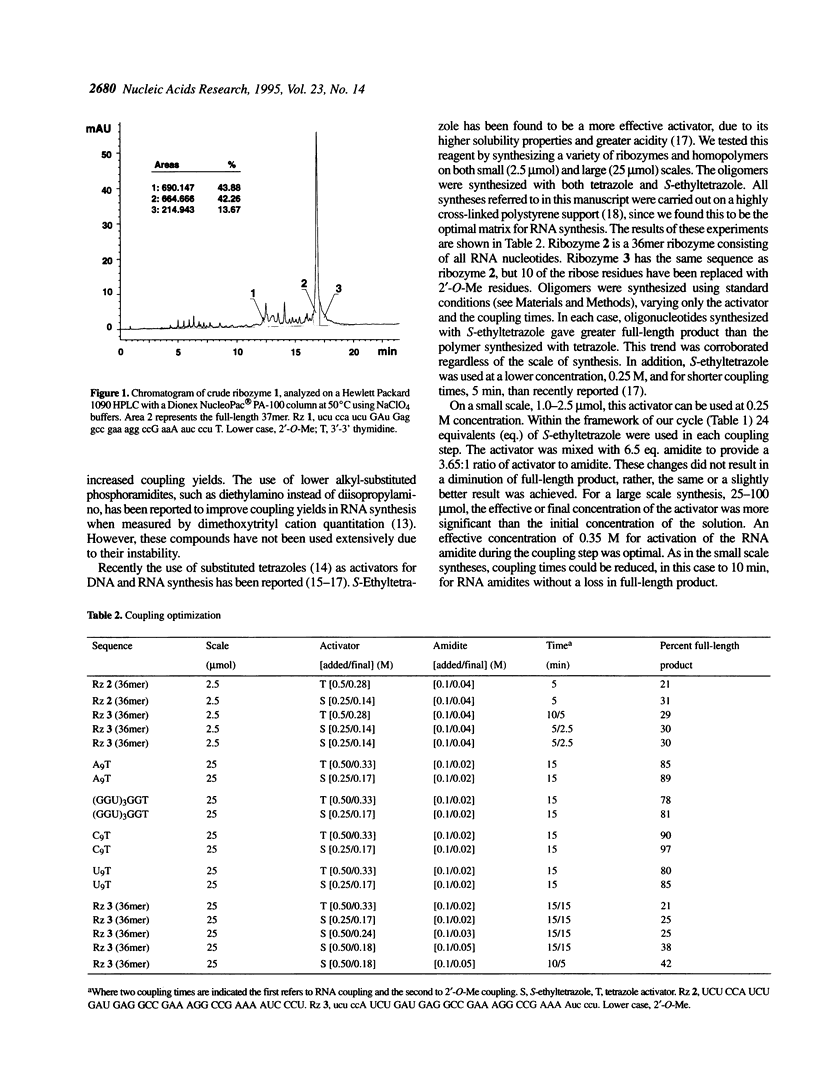
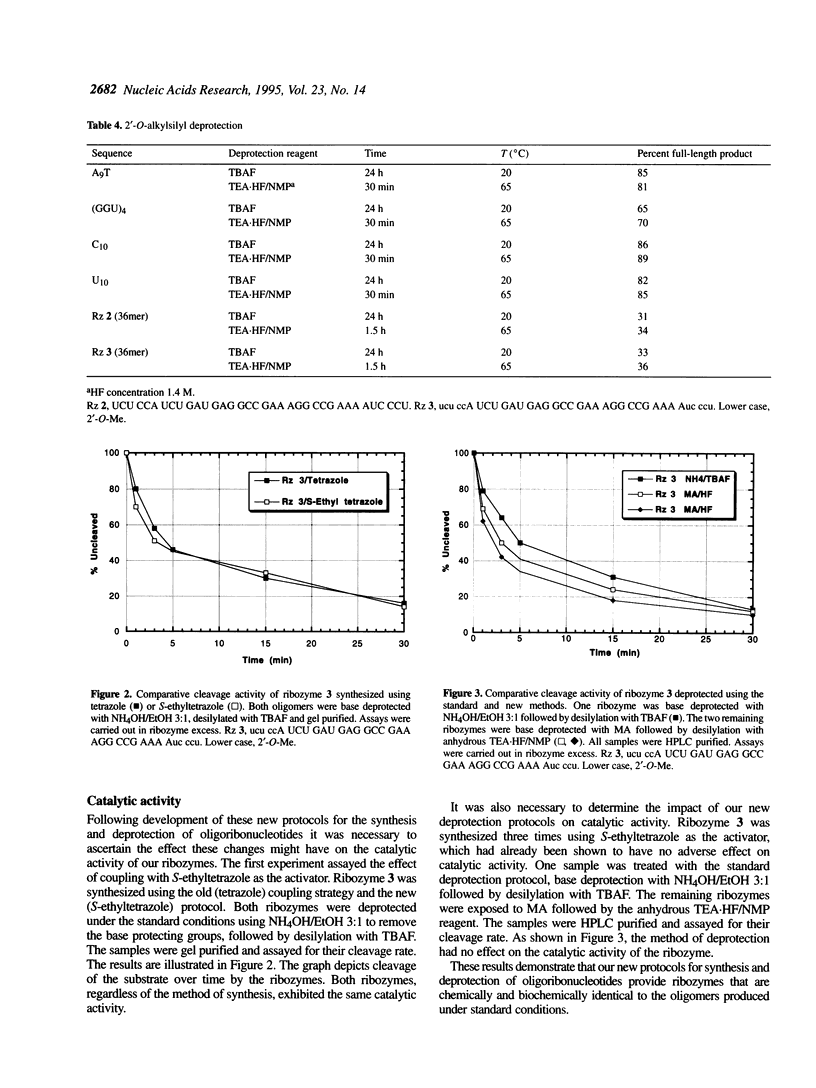
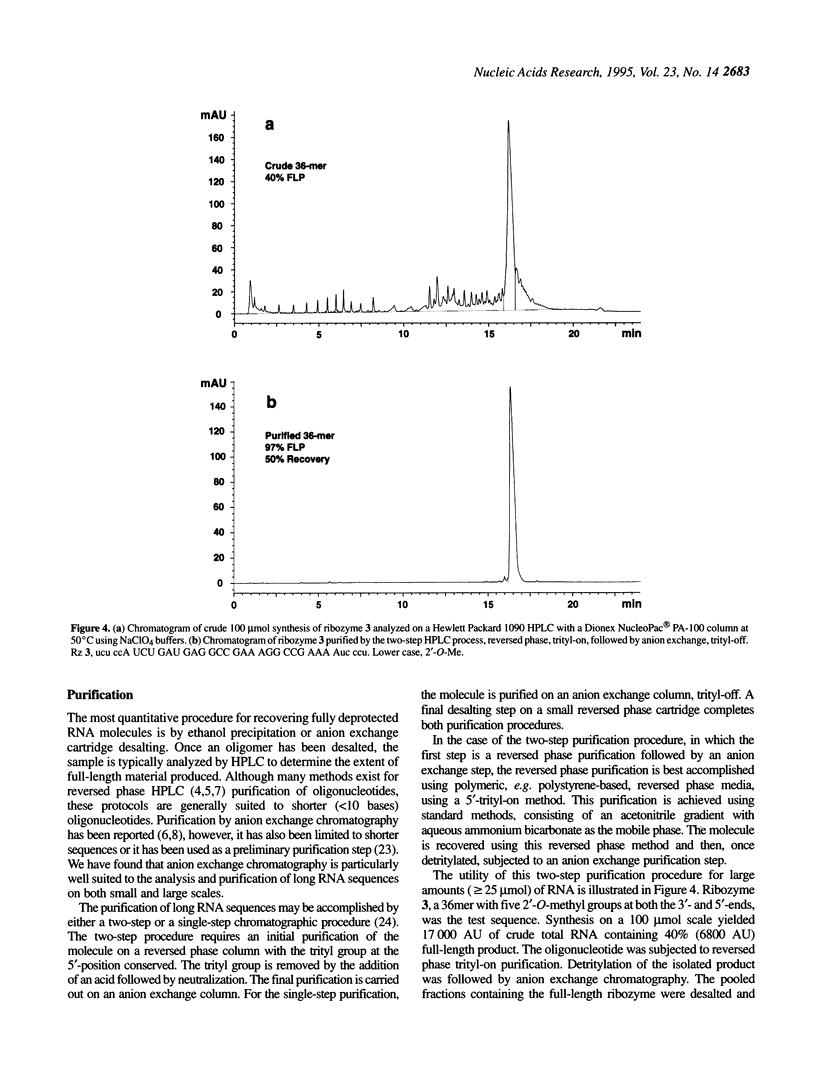
Improvements in the synthesis, deprotection and purification of oligoribonucleotides are described. These advances allow for reduced synthesis and deprotection times, while improving product yield. Coupling times are reduced by half using 5-ethylthio-1H-tetrazole (S-ethyltetrazole) as the activator. Base and 2'-O-t-butyldimethylsilyl deprotection with methylamine (MA) and anhydrous triethylamine/hydrogen fluoride in N-methylpyrrolidinone (TEA.HF/NMP), respectively, requires a fraction of the time necessitated by current standard methods. In addition, the ease of oligoribonucleotide purification and analysis have been significantly enhanced using anion exchange chromatography. These new methods improve the yield and quality of the oligoribonucleotides synthesized. Hammerhead ribozymes synthesized utilizing the described methods exhibited no diminution in catalytic activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaix C., Duplaa A. M., Molko D., Téoule R. Solid phase synthesis of the 5'-half of the initiator t-RNA from B. subtilis. Nucleic Acids Res. 1989 Sep 25;17(18):7381–7393. doi: 10.1093/nar/17.18.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D. J., McLaughlin L. W. Importance of specific adenosine N7-nitrogens for efficient cleavage by a hammerhead ribozyme. A model for magnesium binding. Biochemistry. 1992 Nov 17;31(45):10941–10949. doi: 10.1021/bi00160a001. [DOI] [PubMed] [Google Scholar]
- Gasparutto D., Livache T., Bazin H., Duplaa A. M., Guy A., Khorlin A., Molko D., Roget A., Téoule R. Chemical synthesis of a biologically active natural tRNA with its minor bases. Nucleic Acids Res. 1992 Oct 11;20(19):5159–5166. doi: 10.1093/nar/20.19.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K. B., McLaughlin L. W. Properties of pseudouridine N1 imino protons located in the major groove of an A-form RNA duplex. Nucleic Acids Res. 1992 Apr 25;20(8):1883–1889. doi: 10.1093/nar/20.8.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogrefe R. I., McCaffrey A. P., Borozdina L. U., McCampbell E. S., Vaghefi M. M. Effect of excess water on the desilylation of oligoribonucleotides using tetrabutylammonium fluoride. Nucleic Acids Res. 1993 Oct 11;21(20):4739–4741. doi: 10.1093/nar/21.20.4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M., Ohtsuka E. Effects of phosphorothioate and 2-amino groups in hammerhead ribozymes on cleavage rates and Mg2+ binding. Biochemistry. 1991 May 28;30(21):5145–5150. doi: 10.1021/bi00235a005. [DOI] [PubMed] [Google Scholar]
- Odai O., Hiroaki H., Sakata T., Tanaka T., Uesugi S. The role of a conserved guanosine residue in the hammerhead-type RNA enzyme. FEBS Lett. 1990 Jul 2;267(1):150–152. doi: 10.1016/0014-5793(90)80311-6. [DOI] [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Davis P., Usman N., Pérez J., Hodge R., Kremsky J., Casale R. Labile exocyclic amine protection of nucleosides in DNA, RNA and oligonucleotide analog synthesis facilitating N-deacylation, minimizing depurination and chain degradation. Biochimie. 1993;75(1-2):13–23. doi: 10.1016/0300-9084(93)90019-o. [DOI] [PubMed] [Google Scholar]
- Slim G., Gait M. J. Configurationally defined phosphorothioate-containing oligoribonucleotides in the study of the mechanism of cleavage of hammerhead ribozymes. Nucleic Acids Res. 1991 Mar 25;19(6):1183–1188. doi: 10.1093/nar/19.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman N., Cedergren R. Exploiting the chemical synthesis of RNA. Trends Biochem Sci. 1992 Sep;17(9):334–339. doi: 10.1016/0968-0004(92)90306-t. [DOI] [PubMed] [Google Scholar]
- Usman N., Egli M., Rich A. Large scale chemical synthesis, purification and crystallization of RNA-DNA chimeras. Nucleic Acids Res. 1992 Dec 25;20(24):6695–6699. doi: 10.1093/nar/20.24.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman E., Strömberg R. Removal of t-butyldimethylsilyl protection in RNA-synthesis. Triethylamine trihydrofluoride (TEA, 3HF) is a more reliable alternative to tetrabutylammonium fluoride (TBAF). Nucleic Acids Res. 1994 Jun 25;22(12):2430–2431. doi: 10.1093/nar/22.12.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]



