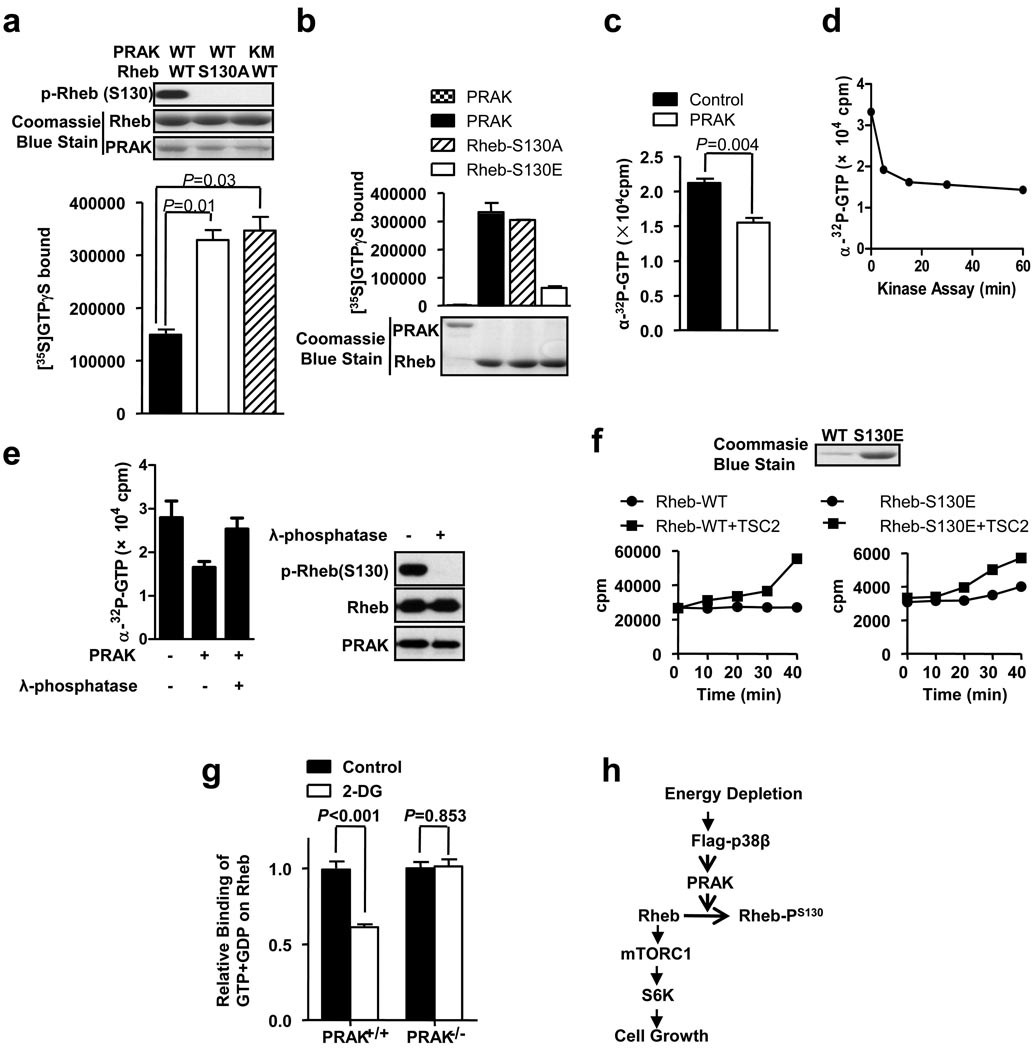
Figure 7.
Rheb phosphorylation by PRAK reduces Rheb’s nucleotide binding ability. (a) Phosphorylation of Rheb in vitro reduces its GTP binding. GST-PRAK or GST-PRAK(KM) was treated with His-p38β plus His-MKK6b(E), and then was purified by GST pull-down. GST-Rheb or GST-Rheb(S130A) was incubated with or without activated GST-PRAK or GST-PRAK(KM) in a kinase buffer containing [35S]GTPγS. The samples were then applied to a GTP loading assay. Error bars indicate mean +/− s.e.m., n=3 samples, Student’s t-test. (b) The phospho-Rheb mimic mutant GST-Rheb-S130E bound much less GTP. GST-Rheb, GST-Rheb-S130A, and GST-Rheb-S130E were applied to a GTP loading assay (upper panel). The amounts of proteins are shown in the lower panel. (c) Phosphorylation of Rheb releases bound GTP. GST-Rheb was loaded with α-32P-GTP first, and was then treated with or without activated PRAK for 60 min at 30 °C. The bound GTP was determined. (d) The reduction of bound α-32P-GTP during Rheb phosphorylation. α-32P-GTP-loaded GST-Rheb was incubated with or without activated GST-PRAK for 0, 5, 15, 30, or 60 min at 30 °C. The bound GTP was determined. (e) Dephosphorylation of phospho-Rheb recovers Rheb’s α-32P-GTP binding ability. α-32P-GTP-loaded GST-Rheb was treated with or without PRAK and then treated with or without λ-phosphatase, as indicated. The bound GTP was determined (left panel). The levels of phospho-Rheb, Rheb, and PRAK were shown in right panel. (f) Rheb-wt or Rheb-S130E was applied in a GTP hydrolysis assay in the absence and presence of HA-TSC2 (left two panels). The Coomassie blue staining of Rheb-wt and Rheb-S130E protein is shown in right panel. (g) PRAK+/+ and PRAK−/− MEF cells were metabolically labeled with 32P-phosphate, then treated with or without 25mM 2-DG for 30 min. Endogenous Rheb was immunoprecipitated. GTP and GDP bound to Rheb was analyzed by thin layer chromatography and quantitated by a Phosphorimager. Data are represented as the mean +/− s.e.m., n=4 samples, Student’s t-test. The relative binding of GTP+GDP on Rheb was calculated by setting the binding of GTP+GDP on Rheb in control groups to 1.0. (h) A proposed model of the p38β-PRAK cascade in regulating the mTORC1 pathway following energy starvation.