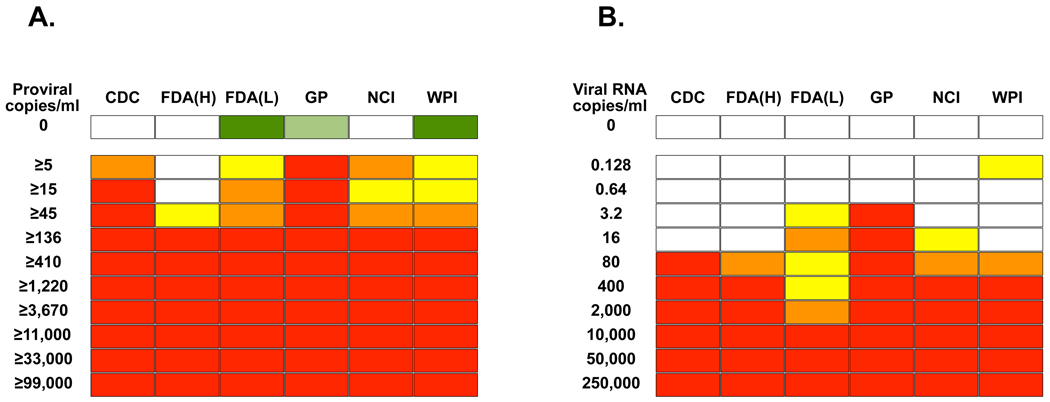
Figure 1. Sensitivity of detection of XMRV by multiple laboratories.
Analytical panels for whole blood (A) and plasma (B) were created by serial dilution of 22Rv1 cells or 22Rv1 cell supernatant in whole blood and plasma respectively. The dilutions were tested in triplicate by six laboratories (CDC, FDA lab of Dr Hewlett [FDA(H)], FDA lab of Dr Lo [FDA(Lo)], Gen-Probe Inc [GP], NCI and WPI). Red represents 3/3 replicates being positive, orange is 2/3, yellow is 1/3 and white is 0/3. Replicates of six negatives were performed and white represents 0/6, while green represents 1/6 replicates being positive. In the case of FDA(Lo) and WPI subsequent sequencing demonstrated in each case that the amplification product in the single false positive result for a negative control sample was of human genomic origin. In the case of GP, a repeat by a separate operator yielded 0/6 controls as positive.