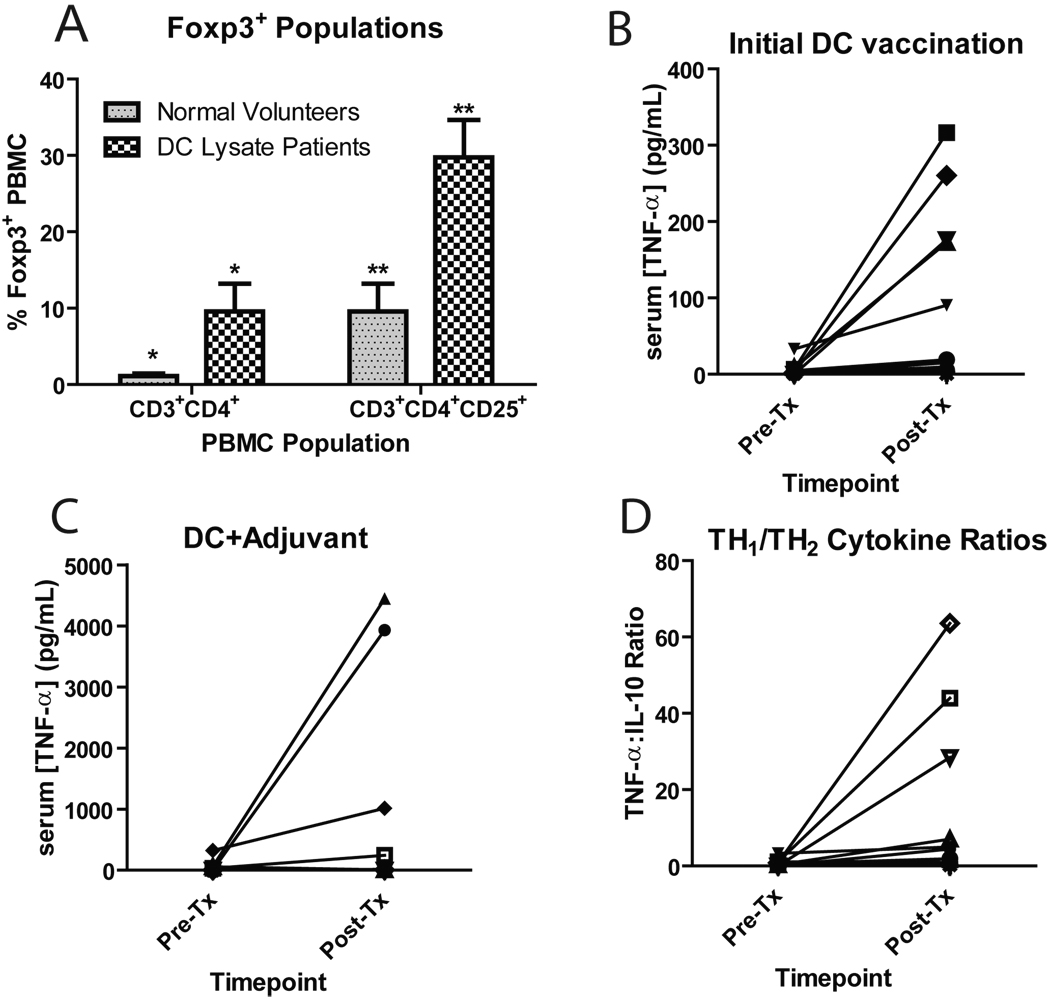
Figure 2.
Peripheral blood immune monitoring data. (A) PBMC’s from normal volunteers and DC trial patient pre-vaccination timepoints were thawed and stained for the expression of CD3, CD4 and CD25, followed by the intracellular labeling of Foxp3. Stained cells were acquired on a BD FacsCalibur flow cytometer and analyzed using FloJo software. The frequencies of CD3+CD4+Foxp3+ and CD3+CD4+CD25+Foxp3+ PBMC’s between normal volunteers and glioblastoma patients enrolled in this trial are compared. (*p=0.04; **p=0.01) (B,C) Serum cytokine responses, measured pre- and day 14 post-vaccination, after the initial course of DC vaccination (B) or after booster DC vaccinations with either 5% imiquimod or poly ICLC (C). Serum from patients enrolled on this clinical trial was thawed, labeled with cytometric bead array (CBA) antibody-coated beads, washed and subjected to analysis on a BD FacsCalibur flow cytometer together with cytokine standards. Quantitative assessment of cytokine levels was accomplished with a Microsoft Excel-based CBA software program. (D) Th1/Th2 cytokine ratios. Raw cytokine data for serum TNF-α and IL-10 at each timepoint were divided to generate a Th1:Th2 ratio.