Abstract
Background
Serotonin is an important neurohumoral molecule in the gut but its signaling system is not fully developed in the neonatal gastrointestinal (GI) tract. This study was aimed to evaluate the postnatal maturation of the serotonin signaling in the small intestine.
Methods
In vitro amperometry for real time measurement of serotonin at the mucosal surface, immunoblot, immunohistochemistry and high-performance liquid chromatography (HPLC) were used to examine serotonin handling in ileal segments from guinea pigs of different ages.
Key Results
Extracellular serotonin levels significantly declined over the first 3 postnatal weeks, after which levels increased and reached its maximum at 9 weeks postnatally. Serotonin levels were insensitive to the inhibition of the serotonin transporter (SERT) until the animals reached 3 weeks old. Measurement of serotonin and its metabolite 5-hydroxyindole acetic acid (5-HIAA) in the mucosa revealed that the serotonin turnover was significantly lower in neonates. Immunoblot and immunohistochemistry showed that SERT expression was extremely low in the neonatal period. Serotonin staining in cross section showed that enterochromaffin (EC) cells were preferentially localized in the crypt region in neonates and the number of EC cells was significantly higher in 9 week old animals.
Conclusions
SERT expression is low in the neonatal intestine and serotonin signaling matures postnatally. Extracellular serotonin levels decrease during the first 3 neonatal weeks as SERT expression increases. Extracellular serotonin levels increase after 3 weeks (weaning) possibly due to an increase in EC cell numbers. Postnatal maturation of serotonin signaling coincides with dietary changes in the developing guinea pig.
Keywords: Postnatal maturation, 5-HT, serotonin, enteric nervous system
INTRODUCTION
Serotonin is an important neurohumoral signaling molecule in the gut (1). About 90% of whole body serotonin content is synthesized and stored in intestinal enterochromaffin (EC) cells (1). EC cells are sensory transducers that respond to mechanical or chemical stimulation of the mucosa by releasing serotonin (2, 3, 4). Serotonin then acts on mucosal endings of enteric primary afferent neurons and extrinsic primary afferent neurons to initiate motor reflexes and intestinal sensation (5). Serotonin released from EC cells is cleared by the serotonin transporter (SERT), which is expressed by enterocytes in the mucosa (6). Serotonin captured by enterocytes is metabolized to 5-hydroxyindole acetic acid (5-HIAA) by monoamine oxidase (7). Normal serotonin signaling from EC cells depends on serotonin clearance by SERT expressed by enterocytes (1, 7).
The gastrointestinal (GI) tract begins its development during the prenatal period and continues to mature postnatally (15, 16). For example, a recent study demonstrated that inhibitory nerves in the enteric nervous system (ENS) are relatively more effective in neonatal guinea pigs (13). Furthermore, the postnatal decline in inhibitory nerve function may be due to the dilution of inhibitory neurons by other neuronal phenotypes (14). Serotonin signaling in the GI tract is also not fully mature at birth. We have previously demonstrated that neonatal guinea pigs express low levels of SERT and have higher extracellular levels of serotonin compared to animals several weeks old (8). A better understanding of this postnatal maturation of serotonin signaling is important because serotonin plays a key role in regulating visceral sensation and gut motility (9, 10, 11). Indeed, serotonin may be a neurotrophic factor for postnatal neurogenesis in the ENS (12) suggesting its possible role in the normal ontogeny of small intestine motility. Thus, immature or impaired development of serotonin signaling could contribute to pediatric gut dysfunction.
Pediatric motility and visceral sensation disorders affect approximately 1.7 million children in the U.S. and health-related costs for treatment of these disorders is almost $4 billion/year (17). GI motility disorders also negatively impact school performance and social development of children and they cause great disruption in family life (18). In this study, we have measured real time serotonin levels in the ileal segments of guinea pigs at neonatal (≤ 48 h), 1, 3, 6 and 9 week postnatal time points. We also used chromatographic, immunohistochemical and immunoblotting techniques to probe the underlying differences in serotonin handling between these age groups. We believe this study provides important insights about the postnatal maturation of the serotonin signaling system in the GI tract.
MATERIALS AND METHODS
Animal Use and Tissue Preparation
Guinea pigs were lightly anesthetized via halothane inhalation, stunned, and exsanguinated by severing the major neck blood vessels. Animal use protocols were approved by the Institutional Animal Use and Care Committee at Michigan State University. Male guinea pigs at neonatal (≤48 hours postnatal) 1, 3, 6 and 9 week postnatal time points were obtained from Emergent Biosolution, Inc. (Lansing, MI. USA). At three weeks postnatal age, the animals are fully weaned and at nine weeks of age, they are considered to have reached puberty (http://netvet.wustl.edu/species/guinea/guinpig.txt). Tissues for all the experiments were prepared as described before (8; 20; 21).
Diamond Microelectrode Preparation and Amperometry
The boron-doped diamond microelectrode was prepared as described previously (19, 20, 21) and amperometric recording was performed according to the protocol established by Zhao et al. 2010 (20). In this study, the current-distance measurement was performed three times and average of three measurements at each distance was calculated for each animal. The current-distance relationship was tested in the presence or absence of a SERT inhibitor, citalopram (1 μM; C7861, Sigma, USA). All data were collected using a multichannel potentiostat (BioStat, Bioscience, Inc., Chelmsford, MA) and area under the current-time curve (AUC) was integrated (i.e., charge) was used for data analysis (see below). The microelectrode was mounted on a micromanipulator (Mitutoyo, Co., Aurora, IL) for precise positioning relative to the tissue. The microelectrode was poised at 0.6 V vs. a Ag|AgCl reference electrode for serotonin detection.
High-Performance Liquid Chromatography (HPLC) Measurements of Tissue Levels of Serotonin and 5-HIAA
The mucosal layer was removed from ileal tissues using a sharp razor blade and then placed into 500 µL of ice-cold 0.1 mol/L perchloric acid. This sample was homogenized and centrifuged at 14,000 g for 10 min. The supernatant and pellet were separated and stored on ice prior to further analysis. HPLC analysis of serotonin and 5-hydroxyindole acetic acid (5-HIAA) was carried out using a CoulArray system (ESA Biosciences). A MD-150 column (150 × 3.2 mm, 3 µm particle size, ESA Bioscience) and mobile phase (90 mmol/L NaH2PO4, 50 mmol/L citric acid monohydrogen, 1.7 mmol/L sodium octyl sulfate, 50 µmol/L EDTA, and 10% acetonitrile) flowing at 0.6 mL/min were used for separation. The detection potential was set at 200 mV with a guard cell that was positioned after the autosampler (model 542, ESA Biosciences) set at 350 mV. The injection volume was 20 µL and the system was kept at 35 °C. The total protein level in the tissue samples was measure by the Lowry method (22).
SDS-PAGE and Immunoblotting Analysis
Mucosal extracts from guinea pig ileum were prepared as described before (8). Equal amounts (50 µg total protein) of the mucosal extract were combined with sample buffer (62.5 mmol/L Tris pH 6.8, 2% w/v SDS, 144 mmol/L β-mercaptoethonal, 10% v/v glycerol and 0.0024% w/v bromophenol blue) and proteins were separated by 10% SDS-PAGE and transblotted to a PVDF membrane. The membrane was blocked with 5 % (w/v) dry milk in 1% Tween-20, 20 mmol/L Tris pH 7.5, 250 mmol/L NaCl (T-TBS) for 2 hours at room temperature. The membrane was then incubated with mouse monoclonal anti-rat SERT (1:100 dilution; Santa Cruz Biotechnology, SC-33724) at 4°C overnight. To determine specificity of this antibody, a 10x molar excess of competing oligopeptide (GTLKERIIKSITPETPTEIPC, rat and guinea pig amino acids 602-622, http://genome.ucsc.edu) was pre-incubated with the primary antibody overnight at 4°C prior to immunoblotting. After washing with T-TBS, the membrane was incubated with goat horseradish peroxidase-conjugated anti-mouse IgG (1:50,000 dilution; Santa Cruz Biotechnology, SC-2005) for 1 hour at room temperature. After another T-TBS wash, the blot was developed using the ECL Plus Western Blotting Detection System (GE Healthcare). All membranes were stained with Coomassie Brilliant Blue R250 to verify equal protein loading and quantitation of total protein in each lane. The intensity of SERT bands and total protein on membranes was quantitated using ImageJ software (http://rsb.info.nih.gov/ij/) and the SERT protein level in each lane (densitometry) was normalized to the total loaded protein.
Immunohistochemistry
Cross sections of guinea pig ileum were prepared as described before (8). Sections were incubated in either mouse anti-SERT (sc-33724, Santa Cruz Biotechnology, USA) or mouse anti-serotonin (ab16007, Abcam Inc, USA) for 24 h at 4 °C (1:200 dilution in PBS). After the excess serum was washed off with 3 × 10 min changes in PBS, the sections were incubated in the FITC-conjugated donkey anti-mouse IgG (Jackson; 715-095-150) for 2 h at room temperature (1:40 dilution in PBS). The sections were washed in PBS for 3 × 10 min and then mounted in buffered glycerol (pH = 8.6) for fluorescence microscopy. Staining was viewed using a Nikon fluorescence microscope (model TE 2000-U), and images were acquired and analyzed using MetaMorph software. Distribution of EC cells was quantified by making measurements on 3 to 4 sections of each sample obtained from 3 to 5 guinea pigs in each age group. A line was drawn across the image at the crypt–villus border. The number of cells on each side of the line was counted and cell counts obtained in sections from each animal were summed to provide a single number for crypt or villus EC cells.
Data analysis
Serotonin levels were expressed as the AUC integrated at fixed electrode-tissue distances between 0.25 mm and 1.0 mm. The serotonin current at the two extreme distances were excluded from the area under the curve (AUC) integration (21; 23). All data are expressed as the mean ± standard error where “n” values refer to the number of animals from which the data were obtained. Data from different treatment groups were compared using Student’s t-test, Wilcoxon signed-rank test or one-way ANOVA where appropriate. P < 0.05 was considered statistically significant.
RESULTS
The body weights of the guinea pigs at neonatal (≤48 hours postnatal), 1, 3, 6 and 9 week postnatal time points were (in grams), respectively: 98.9 ± 1.7 (n = 10), 173.8 ± 10.1 (n = 5), 241.4 ± 3.4 (n = 11), 544.9 ± 13.5 (n = 8) and 644.7 ± 53.6 (n = 6).
Real Time Electrochemical Detection of Serotonin
Serotonin was detected as the oxidation current in real time with the microelectrode poised at 0.6 V vs. a Ag|AgCl (Fig 1). The oxidation current increased as the microelectrode was positioned closer to the tissue with the largest currents at the electrode-tissue distance less than 500 µm (Fig 1A). To quantify the current, the current-distant relationship was plotted and AUC between the distances of 1 mm and 0.25 mm was integrated as a measure of extracellular serotonin (Fig 1B). The serotonin levels for the animals at neonatal, 1, 3, 6 and 9 week postnatal ages were respectively (in pA·mm; n = 5 – 8): 257.8 ± 55.0, 110.9 ± 44.2, 83.0 ± 16.9, 139.8 ± 36.3 and 226.0 ± 59.1. The serotonin levels in the presence of citalopram for the animals at neonatal, 1, 3, 6 and 9 week postnatal ages were (in pA·mm; n = 5 – 8): 199.9 ± 34.5, 122.1 ± 18.7, 141.7 ± 30, 277.4 ± 113.2 and 391 ± 124.5. Citalopram (1 μM), a SERT inhibitor, increased the serotonin signal only in the animals older than 3 weeks of age (Fig 1C; P < 0.05, Wilcoxon signed-rank test, n = 5 to 8).
Figure 1. Real time detection of serotonin in ileal mucosa made from the guinea pigs.
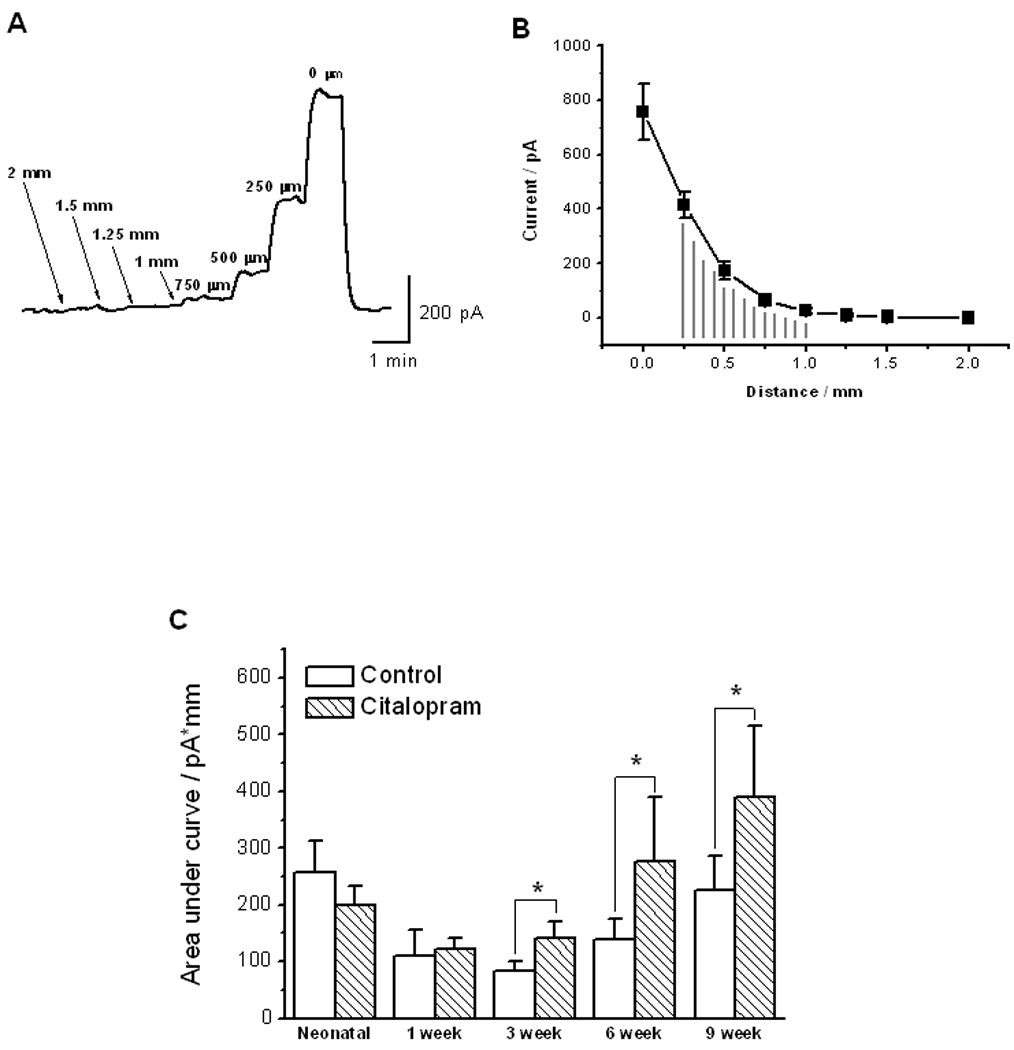
[A]: Representative tracing of serotonin oxidation current from an animal of 1 week old animal. Oxidation current was measured over 10 min as a function of the distance from the tissue to the microelectrode. [B]: Plot of the distance-current relationship for the serotonin oxidation current in the same animal as in [A]. AUC between the distances of 1 mm and 0.25 mm was integrated to reflect the serotonin levels. [C]: Serotonin levels in the presence and absence of citalopram (1 μM) in preparations made from animals at neonatal (≤48 hours postnatal) 1, 3, 6 and 9 week postnatal time points. The serotonin levels decreased from neonatal to 3 week old animals and then increased from 3 week to 9 week old animals. Citalopram (1 μM), a SERT inhibitor, increased the serotonin signal only in the animals older than 3 weeks old (P < 0.05, Wilcoxon signed-rank test, n = 5 to 8). In all experiments, signals were detected by microelectrode at detection potential of 0.6 V vs. Ag|AgCl reference electrode.
HPLC Analysis of Tissue Levels of Serotonin and 5-HIAA
To determine the steady state intracellular serotonin levels in the EC cells, ileal mucosa serotonin was measured using HPLC and normalized with the total protein level in each sample (Fig 2A). Because serotonin captured by enterocytes is metabolized to 5-HIAA by monoamine oxidase (9), the level of 5-HIAA in the ileal mucosa was also determined (Fig 2A). The serotonin levels for the animals at neonatal, 1, 3, 6 and 9 week postnatal ages were (in pg per mg protein; n = 4 – 7): 28.2 ± 9.9, 194.2 ± 91.3, 130.3 ± 57.6, 114.0 ± 39.8 and 90.1 ± 34.8. The 5-HIAA levels for the animals at the same age groups were (in pg per μg protein; n = 4 – 7): 0.6 ± 0.2, 21.3 ± 12.0, 4.3 ± 0.9, 8.9 ± 3.1 and 6.5 ± 10.5. The 5-HIAA/serotonin ratio levels for the animals at the same age groups were (n = 4 – 7): 2.0 ± 0.5, 9.0 ± 1.5, 5.4 ± 1.4, 8.7 ± 0.9 and 7.4 ± 0.5. The 5-HIAA/serotonin ratio was not statistically different between the 1, 3, 6 and 9 week old animals but the ratio in the neonatal group was significantly lower as compared to the other age groups (One-way ANOVA with Bonferroni correction, n = 4 to 7; Fig 2B).
Figure 2. Serotonin and 5-HIAA levels in the ileal mucosa as measured using HPLC.
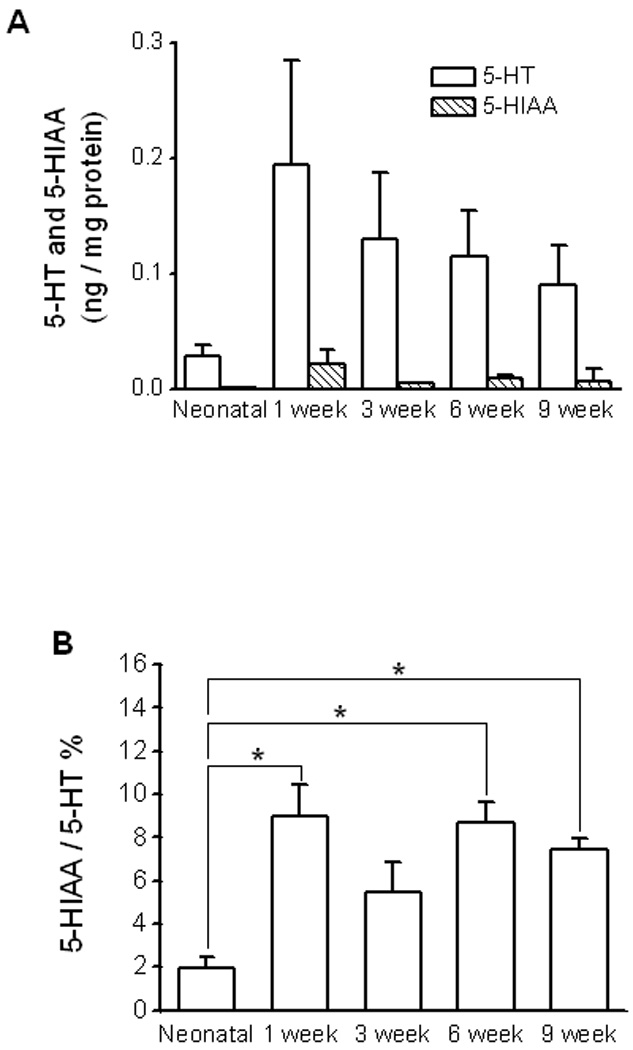
[A]: Summary for amount of serotonin and 5-HIAA in different age groups (n = 4 to 7). [B]: The 5-HIAA/serotonin ratio (serotonin turnover) was very low in neonates. Asterisks indicate statistic significance (One-way ANOVA with Bonferroni correction, n = 4 to 7).
SERT Protein Levels as a Function of Postnatal Age
Immunoblotting of extracts from mucosal scrappings of ileum revealed a band with an apparent molecular weight of ~70 kilodaltons (Fig 3A), consistent with the calculated molecular weight of 70,320 kilodaltons for SERT. No bands were observed with secondary antibody alone or after preincubation with a molar excess of competing oligopeptide (inset, Fig. 3A). SERT protein levels were normalized to the total protein present in each lane and their quantitation values at neonatal, 1, 3, 6 and 9 week postnatal ages were respectively (n = 4): 3.4 ± 0.2, 6.3 ± 0.4, 5.6 ± 0.4, 6.0 ± 0.4 and 5.5 ± 0.4. SERT levels were similar between the animals of ages from 1 week old to 9 week old (Fig 3A, B; n = 4). SERT protein levels, however, were significantly (P < 0.05, One-way ANOVA with Bonferroni correction, n = 4) lower in the neonate as compared to the other age groups (Fig 3A, B).
Figure 3. Ileal SERT protein expression and immunohistochemical localization of SERT in ileal sections from different postnatal ages of guinea pig.
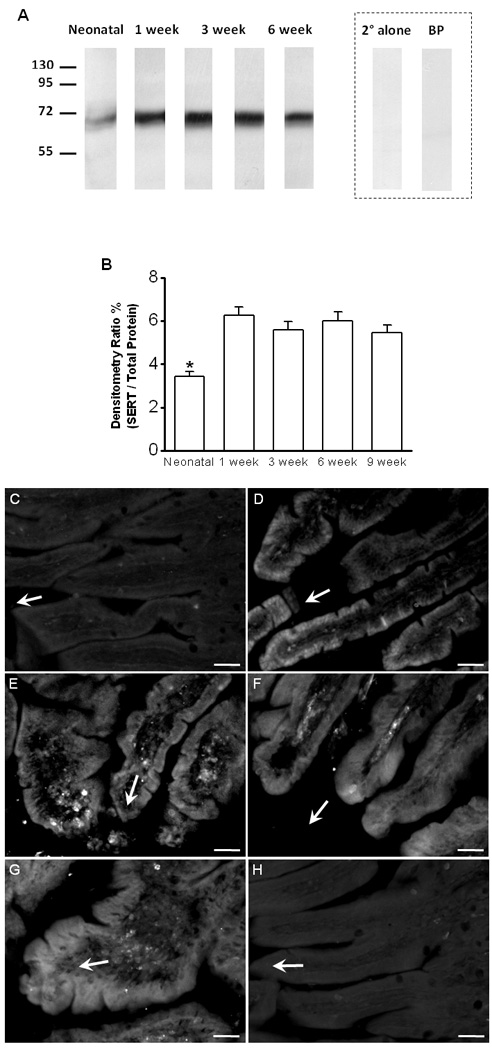
[A]: Representative SDS-PAGE and immunoblot analysis showing SERT protein expression from neonate and 1, 3, 6, and 9 weeks postnatal age. (Inset) Immunoblot with secondary antibody alone (2° alone) and following pre-incubation of the primary antibody with competing oligopeptide (BP; 10x molar excess) prior to immunoblotting. [B]: Intensity of the ~70-kilodalton band was normalized to the total protein present in each lane. The band intensity was significantly lower in the neonate compared to each of the other age groups, but no differences were noted between the animals from age of 1 week old to 9 weeks of age (P < 0.05, One-way ANOVA with Bonferroni correction, n = 4). [C]: SERT-ir in an ileal section from neonatal animal. Minimal amount of SERT-ir was found in enterocytes lining individual mucosal villi. [D]: SERT-ir in an ileal section from an animal of 1 week old. Strong SERT-ir was mainly found in the enterocytes localized on the tips of mucosal villi. [E – G]: Ileal sections from animals of 3 week old [E] 6 week old [F] and 9 week old [G] showing SERT-ir in enterocytes lining several villi. [H]: An example of negative control: omitting the primary antibody removed SERT labeling from an ileal section of a 1 week old animal. [C – H]: Tissues were fixed, processed, stained and camera-captured under the same conditions. Arrows are indicating the tip-direction of the villi. Scale bars: 50 μm.
Immunohistochemistry for SERT
To determine the localization of SERT in the guinea pig ileal mucosa, we used immunohistochemical staining on frozen sections of ileum (Fig 3). In neonates, little SERT immunoreactivity (ir) was observed in the enterocytes lining individual mucosal villi (Fig 3C). In animals at 1 week of age, strong SERT-ir was observed primarily in the enterocytes localized on the tips of mucosal villi (Fig 3D). In ileal sections from animals of 3 weeks to 9 weeks of age, SERT-ir was found evenly distributed in enterocytes lining the mucosal villi (Fig 3E, F and G). No SERT was detected when the primary antibody was deleted from the immunohistochemical protocol (see example in Fig 3H).
Enterochromaffin Cell Distribution
EC cells were found in both villi and crypts on all animals (Fig 4 - E). The serotonin containing cells in neonatal ileum were preferentially localized in the crypt region (Fig 4A). Ileal sections from animals of 1 week old (Fig 4B), 3 week old (Fig 4C), 6 week old (Fig 4D) and 9 week old (Fig 4E) showed that the EC cells were more evenly distributed along crypt-villi axis. Omitting the primary antibody from the immunohistochemical protocol removed localization of serotonin in all tissues (see example in Fig 4F).
Figure 4. Immunohistochemical localization of EC cells in sections from guinea pig ileum.
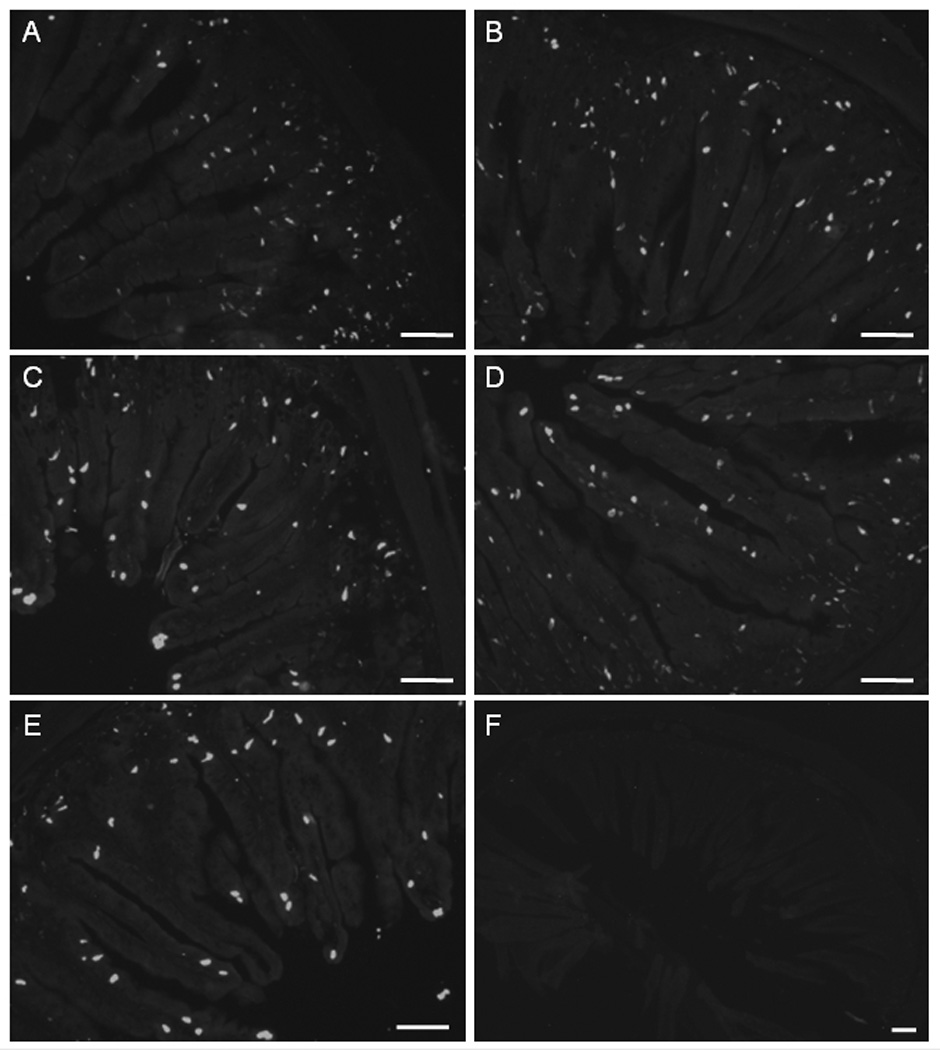
[A]: Section from neonatal ileum showing EC cells preferentially localized in the crypt region. [B – E]: Ileal sections from animals of 1 week old [B], 3 week old [C] 6 week old [D] and 9 week old [E] showing EC cells were evenly distributed along crypt-villus axis. [F]: An example of negative control: omitting the primary antibody removed serotonin labeling from an ileal section of a 1 week old animal. Tissues were fixed, processed, stained and camera-captured under the same conditions. Scale bars: 100 μm.
The numbers of villi in animals at neonatal, 1, 3, 6 and 9 week postnatal ages were (n = 3 to 5): 46.4 ± 3.7, 47.2 ± 1.9, 44.0 ± 5.7, 51.5 ± 1.6 and 50.3 ± 4.1; which did not differ significantly (P > 0.05, One-way ANOVA; Fig 5A). The number of EC cells per each section for the animals at neonatal, 1, 3, 6 and 9 week postnatal ages were (n = 3 – 5): 521 ± 102, 541 ± 34, 571 ± 109, 574 ± 8 and 788 ± 30. The number of EC cells in the 9 week old group was significantly higher (P < 0.05, One-way ANOVA with Bonferroni correction, n = 3 to 5) than in neonates and 3 week old animals (Fig 5B). In the neonates, the number of EC cells in the crypt region was significantly (P < 0.05, unpaired t-test, n = 3 to 5) higher than the number of EC cells in the villi (Fig 5C). The ratio of the number of EC cells in the villus versus the number of EC cells in the crypt was significantly lower (P < 0.05, One-way ANOVA with Bonferroni correction, n = 3 to 5) in neonates (Fig 5D).
Figure 5. Analysis of the distribution of EC cells in the guinea pig ileum.
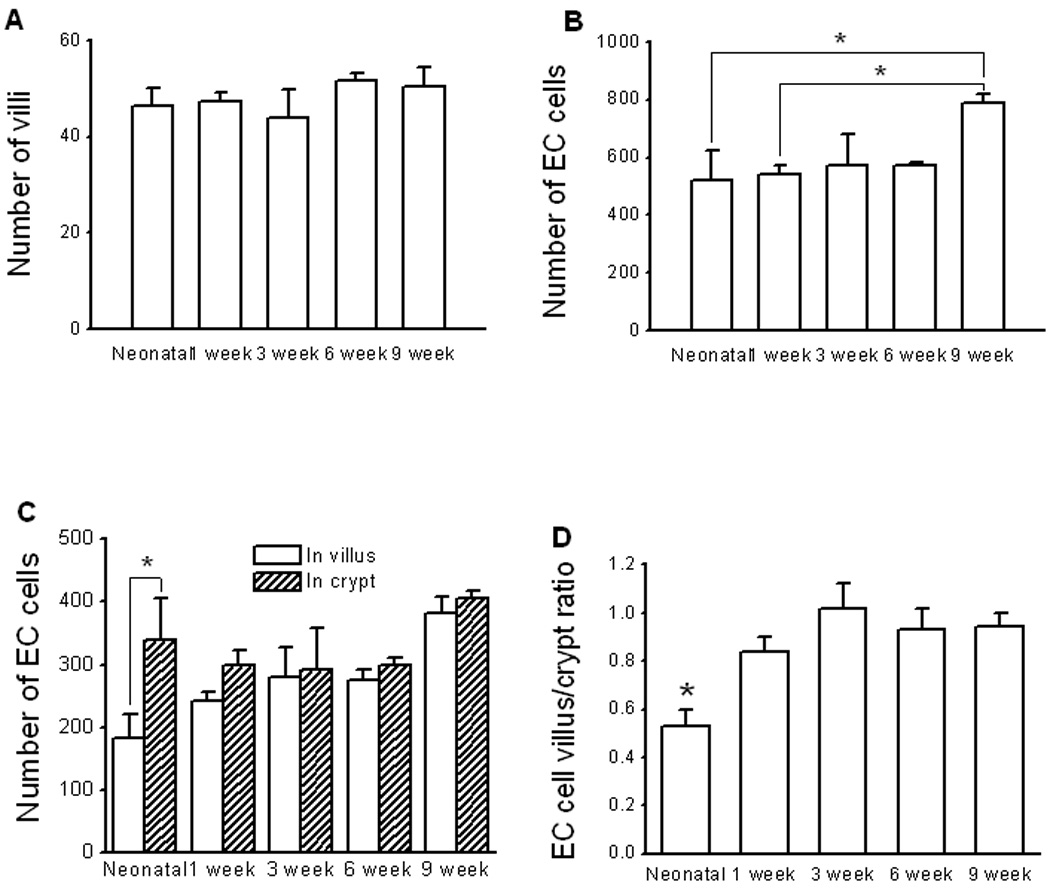
[A]: The total number of villi counted in cross section of ileum from animals of all age groups. [B]: The total number of EC cells in the mucosa counted in cross section of ileum from animals of all age groups. Asterisks indicate statistic significance (One-way ANOVA with Bonferroni correction, n = 4 to 7). [C]: In neonate, the number of EC cells in the crypt was significantly (P < 0.05, unpaired t-test, n = 3) greater than that in the other age groups. [D]: The EC cell crypt-villus ratio was significantly (P < 0.05, One-way ANOVA with Bonferroni correction, n = 3 to 5) larger in neonatal animals.
DISCUSSION
This study is a direct extension of our previous work (8) examining the postnatal maturation of the serotonin signaling system in the GI tract. The GI tract is not fully mature at birth (15, 16) with postnatal functional development adapting to longitudinal growth and dietary changes (24) as well as bacterial colonization (26; 27). Although signaling mechanisms that link the gut lumen environment to the enteric nervous system (ENS) are poorly understood, it has been reported that alterations in the structure and function of the ENS are associated with dietary changes at weaning (25). In the present study, serotonin handling was measured in ileal segments of guinea pig mucosa at several postnatal ages, ranging from the neonatal period to nine weeks of age, and encompasses the weaning period. Our results suggest that an immature serotonin generation-clearance mechanism is present during the neonatal period. For example, extracellular levels of serotonin were elevated and not altered by specific blockade of the serotonin transporter (SERT) when compared to the older animals. Also, serotonin turnover was decreased and SERT protein expression was low in the neonatal period. Furthermore, the serotonin-producing enterochromaffin (EC) cells were localized predominantly in the crypt region in the neonatal period but were found more evenly distributed throughout the entire length of the villus with increased postnatal age.
Extracellular serotonin was measured in real time using amperometry with microelectrodes (8; 19; 20; 21; 28; 29). We specifically used the distance-current relationship between the oxidation current and distance of the microelectrode from the mucosal surface (20). Because serotonin adjacent to the tissue was continuously washed away by buffer in these experiments, a concentration gradient was developed near the recording site. Quantifying the area under the curve (AUC) of the current-distance relationship eliminated artifacts secondary to recording distance. Using this method, we determined that extracellular serotonin was increased in the neonatal period and the oxidation currents of serotonin were sensitive to SERT blockade in all age groups except in the neonates.
Serotonin released from EC cells is transported by SERT into enterocytes and then metabolized to 5-HIAA by monoamine oxidase (6; 7). Therefore, the 5-HIAA/serotonin ratio can be used as a measure of SERT levels and function and intracellular serotonin turnover (8; 30). This study has demonstrated that the 5-HIAA/serotonin ratio is decreased in the neonatal period, consistent with the finding of increased extracellular serotonin based on amperometric measurements and decreased SERT protein levels as determined directly by standard SDS-PAGE and immunoblotting. Interestingly, the immunoblotting analysis revealed a band near 70 kilodaltons in the ileal mucosal extracts from the animals used in this study. This is somewhat different from our previous report which demonstrated a predominant band of ~60 kilodaltons, also from guinea pig ileal mucosal scrapings, but using a different primary antibody (8). A summary of different experimentally reported apparent molecular weights for SERT (calculated MW 70,320) has been recently published (31). It is speculated that the various reported molecular weights may be due to different experimental conditions, different post-translational modifications from various expression systems and species, and the use of different primary antibodies (31; 32).
After the neonatal period, the extracellular level of serotonin decreased between one and three weeks postnatal age and prior to full weaning. This is consistent with our previous observation (8) in which a significantly higher serotonin level was identified in the neonates compared to the animals of three to four weeks of age. This decrease in extracellular serotonin levels prior to weaning is accompanied by an increase in SERT protein expression and a change in EC cell localization at one week of age. In neonates, the EC cells were mainly located in the crypt region of the mucosa. Between the neonatal period and one week of age, however, the proportion of EC cells in the villus increased. The exact physiologic significance of this change in EC cell distribution is unknown at this point. It should be noted that guinea pigs are fully weaned by the fourth postnatal week and they typically have body weights of approximately 300 to 400 grams between the third and fourth week after birth (data provided by our supplier, Emergent Biosolution, Inc., Lansing, MI, USA). The combination of full weaning and a 300 to 400 gram body weight is commonly considered to be that of an "adult" guinea pig (33) and we previously have referred to these guinea pigs as being "adults" (8; 13; 14).
Beyond the neonatal period and beginning at one week of age, we showed that both SERT protein expression and the 5-HIAA/serotonin ratio remained essentially unchanged. However obvious SERT function was not demonstrated until three weeks of age. This conclusion is based on the observation that the serotonin oxidation current was not changed by the inhibition of SERT with citalopram. Therefore, prior to three weeks of age, it is possible that serotonin transport was performed by other monoamine transporters or non-specific cation transporters (34; 35). It is also possible that the nascent SERT was functional to transport yet insensitive to specific SERT inhibitors. This lack of SERT sensitivity to specific phamacologic blockade in the neonatal period of the guinea pig may have important implications to pharmacodynamic and pharmacokinetic properties of SERT inhibitors in the human pediatric population.
Extracellular levels of serotonin increased after three weeks of age when the animals were fully weaned. This increase occurred despite no change in SERT protein expression or serotonin turnover, and the distribution of the EC cells between the crypt and villus remained unchanged. Furthermore, the EC cell number did not increase until nine weeks postnatal age. We cannot exclude the possibility that serotonin release per individual EC cell increased between three and six weeks. Nevertheless, beyond six weeks of age, the increase in extracellular serotonin may be due, at least in part, to an increase in actual EC cell number.
SUMMARY AND CONCLUSIONS
In summary, serotonin signaling in the gut is not mature at birth. A transient period exists at an early postnatal stage during which the serotonin generation-clearance mechanism is not fully developed. We believe extracellular serotonin in the gut decreases during the first three postnatal weeks secondary to an increase in SERT protein expression on the enterocytes. After the animals are fully weaned between three and four weeks of age, extracellular serotonin levels increase possibly due to an increase in the number of serotonin-producing EC cells. The nascent SERT protein present in the neonate may be pharmacologically and functionally different from more mature animals.
ACKNOWLEDGEMENTS
The authors thank Dr. Bhavik Patel for his valuable technical advice.
Grant Support: This work is supported by the National Institutes of Health HD056197 (XB), DK082967 (to GMS) and a M.S.U. intramural grant for new faculty (to MMK).
Footnotes
- Hong Zhao: Amperometry; Real time recording; immunohistochemistry; Data analysis
- Iva Sovadinova: Western Blot; Data analysis
- Vernon M. Swope: Electrode preparation
- Greg M. Swain: Data analysis; Technical support; Manuscript preparation
- Mark M. Kadrofske: Data analysis; Technical support; Manuscript preparation; English proofreading
- Xiaochun Bian: Study design; Data analysis; Technical support; Wrote the paper
References
- 1.Mawe GM, Coates MD, Moses PL. Intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067–1076. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- 2.Bulbring E, Lin RC. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol. 1958 Mar 11;140(3):381–407. 1958. [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci. 1992 Jan;12(1):235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Racké K, Reimann A, Schwörer H, Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res. 1996;73(1–2):83–87. doi: 10.1016/0166-4328(96)00075-7. 1996. [DOI] [PubMed] [Google Scholar]
- 5.Gershon MD. Review article: serotonin receptors and transporters-roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20 Suppl 7:3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 6.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a serotonin transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 8.Bian X, Patel BA, Dai X, Galligan JJ, Swain GM. High mucosal serotonin availability in neonatal guinea pig ileum is associated with low serotonin transporter expression. Gastroenterology. 2007 Jun;132(7):2438–2447. doi: 10.1053/j.gastro.2007.03.103. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004 Jun;126(7):1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Galligan JJ, Parkman H. Recent advances in understanding the role of serotonin in gastrointestinal motility and functional bowel disorders. Neurogastroenterol Motil. 2007 Aug;19 Suppl 2:1–4. doi: 10.1111/j.1365-2982.2007.00970.x. 2007. [DOI] [PubMed] [Google Scholar]
- 11.Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol Motil. 2007 Aug;19 Suppl 2:25–31. doi: 10.1111/j.1365-2982.2007.00965.x. 2007. [DOI] [PubMed] [Google Scholar]
- 12.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. serotonin4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009 Aug 5;29(31):9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bian X, Burda JE, Carrasquillo M, Galligan JJ. Postnatal downregulation of inhibitory neuromuscular transmission to the longitudinal muscle of the guinea pig ileum. Neurogastroenterol Motil. 2009;21:969–977. doi: 10.1111/j.1365-2982.2009.01296.x. [DOI] [PubMed] [Google Scholar]
- 14.Patel BA, Dai X, Burda JE, Zhao H, Swain GM, Galligan JJ, Bian X. Inhibitory neuromuscular transmission to ileal longitudinal muscle predominates in neonatal guinea pigs. Neurogastroenterol Motil. 2010 May 13; doi: 10.1111/j.1365-2982.2010.01508.x. 2010 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giaroni C, Knight GE, Zanetti E, Chiaravalli AM, Lecchini S, Frigo G, Burnstock G. Postnatal development of P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2006 May;50(6):690–704. doi: 10.1016/j.neuropharm.2005.11.015. 2006 Epub 2006 Jan 23. [DOI] [PubMed] [Google Scholar]
- 16.Gershon MD, Thompson EB. The maturation of neuromuscular function in a multiply innervated structure: development of the longitudinal smooth muscle of the foetal mammalian gut and its cholinergic excitatory, adrenergic inhibitory, and non-adrenergic inhibitory innervation. J Physiol. 1973 Oct;234(2):257–277. doi: 10.1113/jphysiol.1973.sp010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liem O, Harman J, Benninga M, Kelleher K, Mousa H, Di Lorenzo C. Health utilization and cost impact of childhood constipation in the United States. J Pediatr. 2009;154:258–262. doi: 10.1016/j.jpeds.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 18.Saps M, Seshadri R, Sztainberg M, Schaffer G, Marshall BM, Di Lorenzo C. A prospective school-based study of abdominal pain and other common somatic complaints in children. J Pediatr. 2009;154:322–326. doi: 10.1016/j.jpeds.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 19.Cvacka J, Quaiserová V, Park J, Show Y, Muck A, Jr, Swain GM. Boron-doped diamond microelectrodes for use in capillary electrophoresis with electrochemical detection. Anal Chem. 2003 Jun 1;75(11):2678–2687. doi: 10.1021/ac030024z. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H, Bian X, Galligan JJ, Swain GM. Electrochemical Measurements of Serotonin (serotonin) Release from the Guinea Pig Mucosa Using Continuous Amperometry with a Boron-Doped Diamond Microelectrode. Diamond & Related Materials. 2010;19:182–185. doi: 10.1016/j.diamond.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel BA, Bian X, Galligan JJ, Swain GM. In Vitro Continuous Amperometric Monitoring of 5-Hydroxytryptamine Release from Enterochromaffin Cells of the Guinea Pig Ileum. Analyst. 2007 Jan;132(1):41–47. doi: 10.1039/b611920d. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265. [PubMed] [Google Scholar]
- 23.Marcelli G, Patel BA. Theoretical modelling to understand the neurotransmission mechanism in the gastrointestinal tract. Conf Proc IEEE Eng Med Biol Soc. 2008. 2008:5548–5551. doi: 10.1109/IEMBS.2008.4650471. 2008. [DOI] [PubMed] [Google Scholar]
- 24.Henning SJ. Postnatal development: coordination of feeding, digestion, and metabolism. The American Journal of Physiology. 1981;241(3):G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- 25.Schafer KH, Hansgen A, Mestres P. Morphological changes of the myenteric plexus during early postnatal development of the rat. The Anatomical Record. 1999;256:20–28. doi: 10.1002/(SICI)1097-0185(19990901)256:1<20::AID-AR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Bailey M, Haverson K, Inman C, Harris C, Jones P, Corfield G, Miller B, Stokes C. The development of the mucosal immune system pre- and post-weaning: balancing regulatory and effector function. Proc Nutr Soc. 2005;64:451–457. doi: 10.1079/pns2005452. [DOI] [PubMed] [Google Scholar]
- 27.Perez PF, Doré J, Leclerc M, Levenez F, Benyacoub J, Serrant P, Segura-Roggero I, Schiffrin EJ, Donnet-Hughes A. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119:724–732. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- 28.Bertrand PP. Real-time detection of serotonin release from enterochromaffin cells of the guinea-pig ileum. Neurogastroenterol Motil. 2004;16:511–514. doi: 10.1111/j.1365-2982.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand PP. Real-time measurement of serotonin release and motility in guinea pig ileum. J Physiol. 2006 Dec 1;577(Pt 2):689–704. doi: 10.1113/jphysiol.2006.117804. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geldof M, Freijer JI, Peletier LA, van Beijsterveldt L, Danhof M. Mechanistic model for the acute effect of fluvoxamine on 5-HT and 5-HIAA concentrations in rat frontal cortex. Eur J Pharm Sci. 2008 Mar 3;33(3):217–229. doi: 10.1016/j.ejps.2007.12.001. 2008. [DOI] [PubMed] [Google Scholar]
- 31.Chamba A, Holder MJ, Barnes NM, Gordon J. Characterisation of the endogenous human peripheral serotonin transporter SLCA4 reveals surface expression without N-glycosylation. J Neuroimmunology. 2008;204:75–84. doi: 10.1016/j.jneuroim.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Coers W, Timens W, Kempinga C, Klok PA, Moshage H. Specificity of antibodies to nitric oxide synthase isoforms in human, guinea pig, rat, and mouse tissues. J Histochem Cytochem. 1998 Dec;46(12):1385–1392. doi: 10.1177/002215549804601207. 1998. [DOI] [PubMed] [Google Scholar]
- 33.Furness JB. The Enteric Nervous System. 1st Edn. Oxford, UK: Blackwell Publishing; 2006. [Google Scholar]
- 34.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001 Aug 15;21(16):6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008 Dec 2;105(48):18976–18981. doi: 10.1073/pnas.0800466105. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


