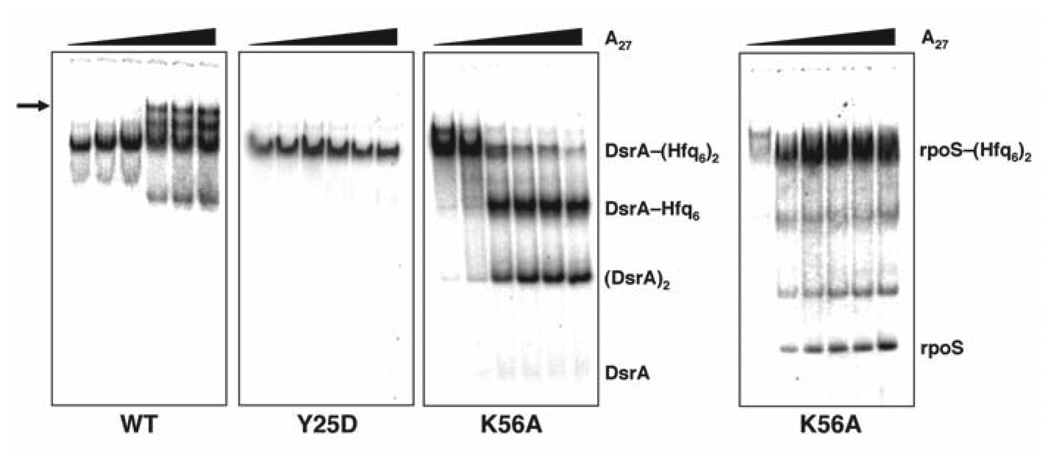
Figure 3.
Native gel analysis showing the effect of A27 addition to the DsrA–(Hfq6)2 complex. DsrA–(Hfq6)2 was preassembled at appropriate concentrations of Hfq (based on the measured affinity constants in Table 2) using 32P-labeled DsrA before the addition of unlabeled A27 (0, 0.03, 0.1, 0.3, 1 or 3 µM). Gels compare the behavior of wild-type (WT) Hfq to Hfq Y25D and Hfq K56A as labeled. A supershifted species is observed (arrow) only in the case of WT Hfq. For Hfq Y25D, the addition of A27 had no effect on DsrA binding. For Hfq K56A, A27 displaced DsrA in the form of the homodimer, DsrA2, based on its gel migration against authentic standards.