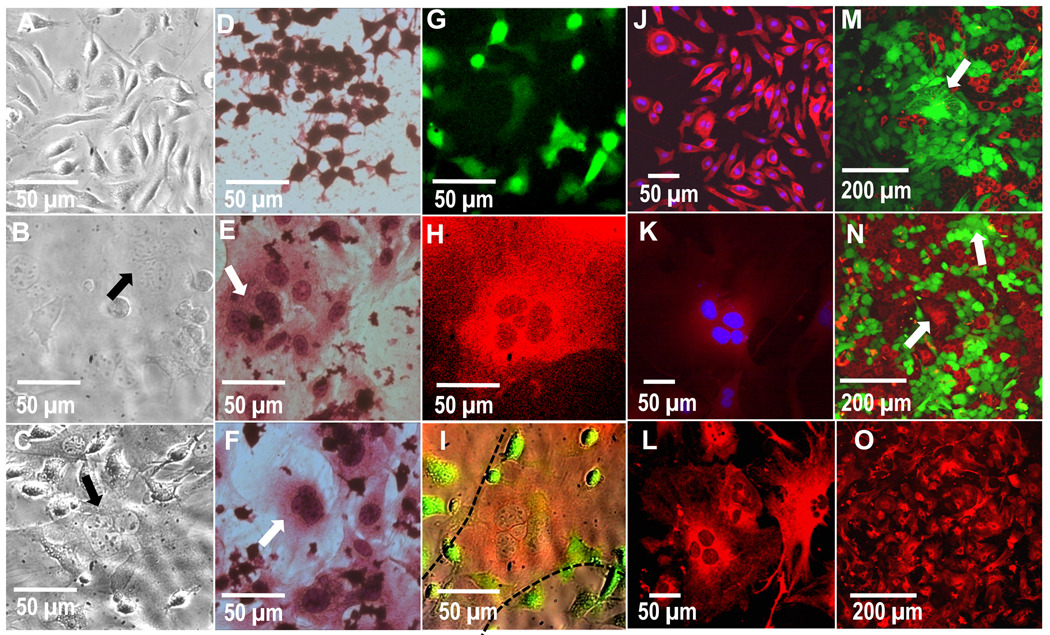
Figure 6.
Phase contrast images of A) primary-derived BMMΦs alone, B) primary fibroblasts alone and C) contact co-culture of BMMΦs and fibroblasts. Note multinucleate cells in both the mono-culture fibroblasts and contact co-cultures (arrows). H&E-stained images showing D) secondary-derived RAWs cultured alone, E) ear fibroblast cultured alone and F) contact co-culture of RAW cells and primary fibroblasts. Multinucleate cells can be seen in fibroblast mono-culture as well as contact co-culture with RAW cells (arrows). G–I) Fluorescent images of contact co-cultured primary macrophages (green channel, G), fibroblasts (Red channel, H) and an overlay of red, green and brightfield channels (I). These images show that the multinucleated cell is red and therefore of fibroblastic origin (H and delineated by dotted lines in I). Confocal images of primary-derived J) BMMΦs and K) fibroblasts labeled with DAPI (blue) and macrophage-marker CD14 (red), revealing a strong CD14 stain in macrophages but not fibroblasts. L and O) show 40X and 20X confocal images, respectively, of primary-derived fibroblasts labeled with fibroblast-marker vimentin (red), showing that the majority of cells in this culture stain positive for fibroblasts, including multinucleate cells. M and N) Confocal images of mono-cultured primary fibroblasts pre-labeled with red or green long-lived intracellular fluorescence prior to culture, where the multinucleated cells (arrows) found were either green or red, but not both (representative images from 3 separate experiments and a total of 9 replicates). This lack of co-localization indicates an absence of fusion to produce cell multinucleation. Images are shown after 3 days of culture.