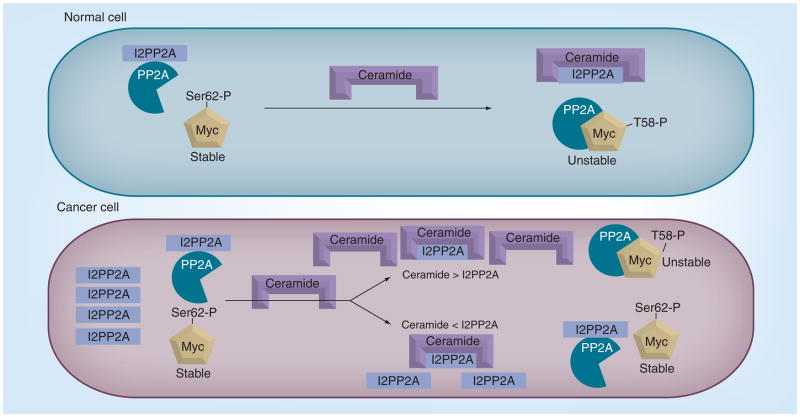
Figure 4. Regulation of oncogenic c-Myc by protein phosphatase 2A via control of ceramide in normal and cancer cells.
In normal cells, ceramide and its binding protein, I2PP2A, which is the inhibitor for PP2A, are mostly in a 1:1 ratio. Therefore, it is believed to be the binding and inactivation of ceramide by I2PP2A that liberates the active form of PP2A, which, in turn, acts upon c-Myc, leading to the dephosphorylation and degradation. In cancer cells, elevated levels of I2PP2A were observed, which inhibits most of the available PP2A and results in stable (active) oncogenic c-Myc. The stable form of c-Myc can mediate tumor growth and cancer progression by upregulating expression of several oncogenes.
I2PP2A: Protein phosphatase 2A inhibitor 2; PP2A: Protein phosphatase 2A.