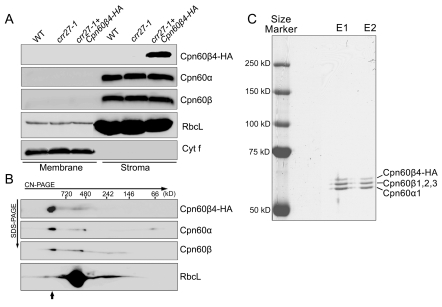
Figure 3. Analysis of the Cpn60β4 subunit.
(A) Cpn60β4 is localized to the chloroplast stroma. Freshly isolated chloroplasts from various genotypes were separated into membrane and stromal fractions. Immunoblot analysis was performed using the indicated antibodies. RbcL and Cyt f were detected as loading and fractionation controls. (B) Stromal protein complexes isolated from crr27-1 complemented by Cpn60β4-HA were separated by CN-PAGE, followed by 2-dimensional SDS-PAGE. The proteins were immunodetected with specific antibodies. A short arrow indicates the position of the Cpn60 complex. (C) Heterooligomeric complex formation between Cpn60β4, Cpn60α1, and Cpn60β1–β3. Chaperonin complex containing Cpn60β4 was purified from the crr27-1 mutant plants expressing HA-tagged Cpn60β4 using the µMACS HA isolation kit. After elution, total proteins were separated by 7.5% SDS-PAGE and stained with CBB. The signals were quantitatively analyzed with Imagemaster software (Amersham Pharmacia Biotech). The stoichiometry of Cpn60β4, Cpn60β1–β3, and Cpn60α1 in the specific chaperonin complex containing Cpn60β4 was estimated to be 17:37:46 and 14:39:47 in the two independent purifications (E1 and E2), respectively.