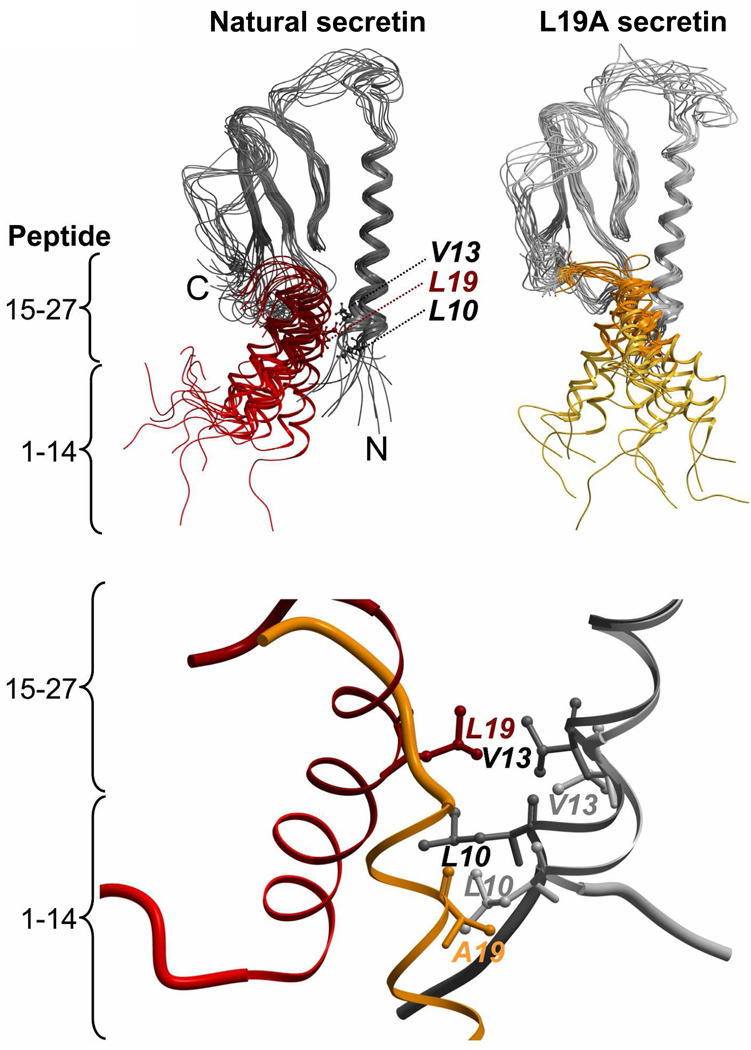
FIGURE 5.
Structural snapshots of the complex of secretin with the amino-terminal domain of its receptor during the 12 ns simulation. Top, superimposed structure for every 1-ns time point in the molecular dynamics simulation for the natural secretin peptide (red, left) and the L19A variant of secretin (gold, right) bound to the receptor amino terminus (shades of black and gray). The complexes started with both peptides in the same conformation. The receptors are colored black (with natural secretin docked, left) and gray (with the L19A variant docked, right), with the regions of the peptides representing amino-terminal residues 1–14 (lighter colors) and carboxyl-terminal residues 15–27 (darker colors) identified. Bottom, a structural snapshot taken at the identical time point (10 ns) in the simulations showing the approximation of residue 19 of the peptides with Leu10 and Val13 of the secretin receptor.