Abstract
We have previously shown that, in early stages of Parkinson's disease (PD), patients with higher reaction times are also more impaired in visual sequence learning, suggesting that movement preparation shares resources with the learning of visuospatial sequences. Here, we ascertained whether, in patients with PD, the pattern of the neural correlates of attentional processes of movement planning predict sequence learning and working memory abilities. High density Electroencephalography (EEG, 256 electrodes) was recorded in 19 patients with PD performing reaching movements in a choice reaction time paradigm. Patients were also tested with Digit Span and performed a visuomotor sequence learning task that has an important declarative learning component. We found that attenuation of alpha/beta oscillatory activity before the stimulus presentation in frontoparietal regions significantly correlated with reaction time in the choice reaction time task, similarly to what we had previously found in normal subjects. In addition, such activity significantly predicted the declarative indices of sequence learning and the scores in the Digit Span task. These findings suggest that some motor and non motor PD signs might have common neural bases, and thus, might have a similar response to the same behavioral therapy. In addition, these results might help in designing and testing the efficacy of novel rehabilitative approaches to improve specific aspects of motor performance in PD and other neurological disorders.
Keywords: Choice Reaction Time, Sequence Learning, Reaching, Working Memory, Event Related Spectral Perturbation
Introduction
It is now well known that Parkinson's disease (PD) affects not only motor functions but also the learning processes involved in different types of motor and non motor tasks (Carbon et al. 2010; Doyon 2008; Ghilardi et al. 2003). However, the relationship between motor and learning deficits has not been thoroughly explored. In a recent study, we specifically addressed this issue (Marinelli et al. 2010) by testing patients in the early stages of PD with a choice reaction time (RT) reaching task and a visual sequence learning test. Both movement preparation and learning skill were affected in patients with PD compared to age matched normal controls. Most importantly, we found that patients with higher reaction times at the motor task were more impaired in the visual sequence learning test. These results were confirmed also using a visuomotor sequence learning task (Ghilardi et al. 2004). Interestingly, we did not find any such correlation in the control group likely due to the small range of variability and to the ceiling effect in learning. In fact, the majority of the control subjects achieved a full learning score. We speculated that movement preparation and sequence learning share important cognitive resources, namely attention, and thus, overlapping neural substrates, likely the frontoparietal network. This could be evident only in the patients’ group because of the larger variability.
A growing body of evidence suggests that modulation of attention resources predicts the speed and accuracy of visual and visuomotor processing (Klimesch et al. 2008; Serences and Yantis 2006; van Dijk et al. 2008). Expectations might enhance perception through adequate allocation of attention, which is promoted by interactions among the frontoparietal regions (Capotosto et al. 2009). Brain rhythms over the posterior areas in the time preceding the appearance of salient stimuli are neurophysiological markers of these processes, as attenuation of power in the alpha and beta bands correlates with reaction time (Klimesch et al. 1998; Sauseng et al. 2005; Thut et al. 2006; Zhang et al. 2008). We recently confirmed these findings in normal subjects performing a choice RT reaching task, the same used in Marinelli et al. (2010): oscillatory activity preceding the presentation of a target stimulus significantly correlated with the latency of movement onset (i.e. the higher the electroencephalographic activity the lower was the reaction time). This correlation was evident on the scalp electrodes overlaying the frontoparietal regions and, most importantly, was specific for the time interval before stimulus presentation (Moisello et al. 2009b).
Based upon these findings, this prestimulus frontoparietal oscillatory activity can be considered as a neural marker of the attentional resources shared by movement preparation and sequence learning. More specifically, decreased top down attention modulation in PD can cause increased reaction time and poorer learning skills: this should be reflected in a strong relationship between EEG activity and specific behavioral indices. We hypothesize that patients with longer movement preparation time and poorer sequence learning will display reduced EEG oscillatory activity before the stimulus appearance over the posterior regions. If this is the case, we also expect the same patients to show lower scores in a well established test of attention and working memory (WM), without a motor component, such as the Digit Span (Wechsler 1997).
The main scope of this paper is to determine the neural correlates of attentional processes involved in both motor and cognitive tasks. Therefore, we focused our study on patients with PD, as they show a wider range of kinematic and cognitive measurements, while we failed to detect significant relationships between learning scores and reaction time in the normal population, likely due to ceiling effects (Marinelli et al. 2010). Thus, we tested a group of individuals affected by PD with a choice RT reaching task, a visuomotor sequence learning test and backward and forward versions of the Digit Span task. The prestimulus EEG activity recorded in the motor task was then correlated with the behavioral performance and correlation scalp maps were produced.
Methods
Subjects
Fifteen right handed patients (nine men and six women, mean age ± SD: 63.9 ± 8.9 years) with the diagnosis of PD UK Brain Bank Criteria (Gibb and Lees 1988) were recruited from the New York University Movement Disorders Outpatients’ Centre. They were in Hohen and Yahr (Hoehn and Yahr 2001) stage from1 to 3 (mean: 1.9± 0.7) and their mean Unified Parkinon's Disease Rating Scale (UPDRS III, motor part; (Fahn and Elton 1987)) score was 18.3 (± 7.7). They had a mean disease duration of 6.9 years (±4.9) and an average Mini Mental Examination Score (Folstein 1989) of 29.2 (±1.3). All subjects underwent structural MRI without contrast to exclude potential structural brain lesions (e.g., vascular lesions, mass lesions, hydrocephalus, cortical atrophy) or signs suggesting atypical parkinsonism. Written informed consent was obtained from all participants under a protocol approved by the institutional review board of the participating institutions.
Patients were tested in “on” state under their regular pharmacological treatment. The tests included two motor tasks, i.e., a choice reaction time task (RAN) and a visuomotor sequence learning task (SEQ), and the Digit Span test forward (DS/FW) and backward (DS/BW), a neuropsychological tool to measure attention and working memory abilities (Wechsler 1997). In all subjects, high density EEG was recorded during RAN.
Motor tasks
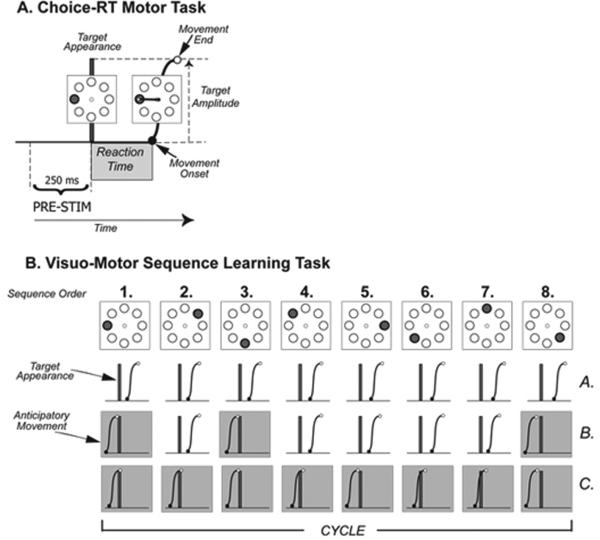
The two motor tasks have been described in details in previous works (Ghilardi et al. 2003, 2008; Moisello et al. 2009a). Briefly, in both, subjects performed reaching movements by moving a cursor with their right hand from a common starting point to one of eight targets (distance = 4 cm) that appeared on a screen at a regular time interval.
In the RAN task (Figure 1 A), targets were presented in unpredictable order at a 3 second interval. Instructions were to reach the target as soon as possible, minimizing reaction time but avoiding anticipation. Subjects performed a block of 96 movements.
Figure 1.
A - The time line for a single trial of the choice RT motor task with representation of the target array. B – Schematic illustration of the development of anticipatory movements during sequence learning. Target are presented at constant time interval of 1.5 s so that temporal occurrence is predictable. At the beginning (A.) movements initiate after target appearance in the course of learning; (B>) movements start becoming anticipatory (grey boxed hand path). Finally, when the sequence is entirely known (C.), all the target appearances are anticipated.
For each response, we computed the reaction time (RT), as the time between stimulus appearance and movement onset, and the movement time (MT), as the time between the onset of the movement and the reversal in the target.
In the SEQ task (Figure 1B), targets were presented in a repeating sequence of eight elements, at 1.5 second intervals. Subjects were explicitly informed that a sequence was presented, they were instructed to learn the order of appearance while reaching for the targets, and to reach the target at the time of its appearance as soon as they knew the order of presentation. After they performed one block of 56 movements (for a total of 7 presentations of the entire sequence, or 7 cycles), subjects were asked to report the order of the sequence verbally and declarative scores were computed. In addition, we computed the number of anticipatory movements, i.e. those movements whose onset time was below the minimum value of reaction time in RAN blocks. This number is strongly correlated with the declarative knowledge of the sequence, both in healthy subjects and patients’ populations (Ghilardi et al. 2003, 2008; Moisello et al. 2009a) and represent an index of declarative learning.
Digit span test
The Digit Span is a task that assesses individual memory span - the longest list of items that a subject can repeat back immediately after presentation. In the DS test the items are numbers that are presented vocally in string of linearly increasing size (2-3-4-5 etc.). The test has two different versions: the forward and the backward. In the former, the subject is asked to repeat the numbers in the same order they were presented while in the latter numbers must be recalled in the reverse order. The DS/FW is widely considered a task of short-term memory and attention. The DS/BW is thought to tap working memory abilities instead, since it requires active manipulation of information hold in memory.
EEG
During the RAN motor task and other motor tasks, EEG activity was recorded by means of high density EEG system (256 electrodes; Hydrocel net, Electrical Geodesics Inc.). EEG signal was referenced to a Cz sensor. Data were collected using the high impedance amplifier Net Amp 300 and Net Station 4.3 (Electrical Geodesics Inc.). Impedances were kept below 50 kΩ with a sampling rate of 250 Hz. EEG data recorded during the execution of all the different tasks were used in the preprocessing stage of the analysis.
Preprocessing
A preprocessing procedure was performed on the continuous data with NetStation 4.3 software (Electrical Geodesics Inc.) and the public license toolbox EEGLAB (Delorme and Makeig 2004) After the exclusion of the channels located on the neck and cheeks, 183 site scalp electrodes were selected and used for further analysis. In the first step, the continuous EEG signal was high pass filtered (Kaiser type finite impulse response filter) above 0.5 Hz and low pass filtered below 80 Hz (band stop at 60 Hz). Bad channels were visually identified and interpolated (spherical splines). The continuous EEG signal was then segmented into epochs of 3750 ms based on movement onset latencies (from 1250 ms before to 2500 after the movement onset). Visual rejection of epochs contaminated by non stereotyped artifacts followed. Of note, stereotyped artifacts like blinks, eye movements and motion related signals were kept in the epoched files to be estimated and removed by Principal Component Analysis (PCA) technique.
In order to identify and remove the extra brain artifactual stereotyped sources from the EEG signal, Principal Component Analysis (PCA) was applied on a single trial basis (Dien and Frishkoff 2005). For a good segregation of the artifactual components from the physiological signal, a sufficient number (~ 10 minutes) of samples is needed (Delorme and Makeig 2004). Therefore, the processed data from the EEG recorded during the same session in several motor tasks were first merged and then submitted to the PCA. Eighty linearly independent spatial filters were obtained. The weights and sphere matrices derived from the calculation were then applied to each of the single epoched files. The PCA processed EEG data were then visually inspected and the components accounting for the eye, muscle, motion related and other kinds of artifacts (eg. line noise) were removed from each file.
Since the initial hypothesis was to investigate the relation of attention modulation in a choice reaction reaching task and individual WM abilities we focused our analysis on the brain activity elicited by the RAN task. Following the preprocessing and the PCA cleaning procedures, an average number of 87.9 (±3.7) out of the initial 96 trials were used. All the results described in this paper were hence derived specifically from RAN block.
Event Related Spectral Perturbation
For each subject, individual Event Related Spectral Perturbation (ERSP) was obtained by measuring the mean event related changes in the power spectrum at all the channels over time with respect to a baseline period. A Fast Fourier transform with Hanning tapers was used in the frequency range between 2 and 35 Hz (for a detailed description of the adopted method see (Delorme and Makeig 2004). Given the relatively short inter trial interval and the variability across trials and subjects, the baseline period was selected on the basis of the single subject's motor performance. To do so, for each subject we estimated the mean latency for the end of the reaching movement and the mean time of stimulus presentation. Based upon these two variables, we determined the individual time window in which the subjects were completely motionless. Baseline period was set to last for 300 ms and ended 250 ms before the target appearance. The window of interest (Prestimulus) instead was set as the period going from 250 ms before to stimulus onset.
After the signal decomposition, individual ERSPs were averaged across the time window of interest and 5 frequency ranges. The frequency ranges were selected as follows: theta (4-8 Hz), alpha1 (8-10 Hz), alpha2 (10-12 Hz), beta1 (12-18 Hz), beta2 (18-25 Hz).
To produce scalp map of the correlation r values, the time/frequency mean individual ERSPs at each electrode was then correlated (Pearson index) with the individual mean reaction time in RAN task, the number of correct anticipatory movements and declarative scores of the SEQ task and the scores of the forward and backward DS task.
Results
Behavior
All patients performed straight movements to the presented targets in both RAN and SEQ tasks. The performance measurements of RAN and SEQ tasks and those of the Digit Span test are summarized in table I. Briefly, in SEQ, the number of anticipatory movements increased during the block (Effect of Cycle: F(6,84)=6.64, p<0.001) and, as in previous works, the total number was positively correlated with the verbal score collected at the end of the block (p<0.001), indicating that both measurements are indices of declarative knowledge of the sequence order. Confirming and expanding previous results in a purely visual learning task (Marinelli et al. 2010), we found that the reaction time measured in RAN was significantly correlated with both the verbal score (p=0.006) and the number of anticipatory movements (p=0.02) in SEQ, a visuomotor sequence learning task: the lower reaction time in the RAN task the better were the subjects at learning the sequence order (see table II for r values). On the other hand, MT, which was moderately correlated with reaction time (p=0.05), did not show any relation to the learning indices, suggesting that attentional processes contribute differently to their variability. Moreover, as reported in table II, we also found significant correlations between reaction time, anticipatory movements, verbal scores and the scores of the forward and backward DS, suggesting that attention is an important component in the acquisition of the sequence order and motor planning.
Table I.
behavioral findings
| TASKS | |||
|---|---|---|---|
| RAN | Mean | S.E. | Range |
| RT (ms) | 312.4 | 6.5 | 248.8 – 376.0 |
| MT (ms) | 462.3 | 24.6 | 289.0 - 589.4 |
| SEQ | |||
| AM (%) | 28.8 | 5.9 | 0 - 80.4 |
| VS (%) | 34.2 | 8.8 | 0 - 8 |
| WM | |||
| DS/FW | 9.8 | 0.6 | 6 - 15 |
| DS/BW | 6.9 | 0.5 | 6 - 12 |
Table II.
correlation between behavioral indices
| RT | MT | AM | VS | DS/FW | DS/BW | |
|---|---|---|---|---|---|---|
| RT | 1 | --- | --- | --- | --- | --- |
| MT | 0.52^ | 1 | --- | --- | --- | --- |
| AM | -0.57^ | -0.30 | 1 | --- | --- | --- |
| VS | -0.67* | -0.30 | 0.82* | 1 | --- | --- |
| DS/FW | -0.70* | -0.43 | 0.58^ | 0.77* | 1 | --- |
| DS/BW | -0.77* | -0.34 | 0.62* | 0.73* | 0.82* | 1 |
in bold the significant correlations
p<0.05
p<0.01
Correlation between EEG activity and performance
We have previously found, using a region of interest approach, that, in young normal subjects, the activity in the beta frequency range (12-25 Hz) that precedes the stimulus appearance was significantly correlated with reaction times, and not with any other kinematic characteristic of the movements. Such activity was distributed over the frontoparietal network (Moisello et al. 2009b). Interestingly, reaction time did not correlate with the activity in any other time window, including the interval between the stimulus appearance and movement onset. Therefore, here, we first estimated the spatial and frequency distributions of the neural activity preceding the stimulus and its relation to reaction time. Then, we determined whether this activity could predict sequence learning and working memory abilities.
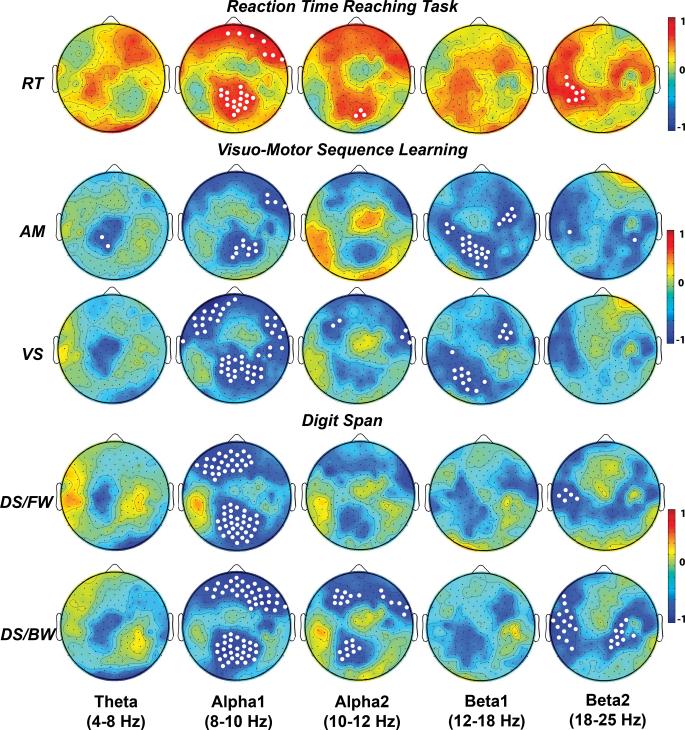
In the prestimulus interval, electrodes over the frontoparietal network showed a decrease of power in both alpha and beta frequency bands when compared to the baseline. Figure 2 shows the scalp maps of the r values obtained by correlating the power variation in the prestimulus interval and for the different frequency bands with the individual reaction time and learning/WM indices (white dots indicate electrodes with p<0.01).. Similar to our previous findings in normal subjects, we found in patients with PD that the oscillatory activity preceding the stimulus was positively correlated with reaction times, with a larger involvement of the lower alpha and higher beta bands. Specifically,. while the relation with alpha band was localized bilaterally over the medial centroparietal electrodes, the correlation with beta band was confined to lateral scalp sites surrounding the left parietal cortex.
Figure 2.
Scalp topographies of the r values (Pearson index, 2 tailed test) obtained from the multiple correlation analysis. RT, Reaction Time; AM, Anticipatory Movements; VS, Visual Score; DS/FW, Forward Digit Span; DS/BW, Backward Digit Span. The white spots indicate electrode sites that showed significant correlations, p<0.01.
For all the investigated frequencies, the scalp topography of correlations with sequence learning and working memory indices was similar to the one obtained with reaction time. However, it is worth noting that low alpha rhythm recorded at frontal sites showed stronger relation with indices that rely mostly on the verbal domain (VS, DSFW, DSBW). In addition, significant correlations in the low beta range over left parietal electrodes and right frontal scalp sites were found only in visuomotor sequence learning task.
The main interest in the current investigation was the posterior alpha rhythm (index of attentive modulation) and the degree to which it is correlated with selected behavioral variables. A remarkable finding is that, among all, the only group of electrodes that showed consistent correlations across the different motor and cognitive indices was located over the parieto occipital scalp region. Table III summarizes the r values for a common representative electrode.
Table III.
correlation between POz alpha1 prestimulus activity and behavioral indices
| RT | AM | VS | DS/FW | DS/BW | |
|---|---|---|---|---|---|
| POz (alpha1) | 0.64 | -0.66 | -0.68 | -0.68 | -0.82 |
in bold the significant correlations, p<0.01
In addition, given the moderate correlation we found between movement and reaction time (table II), we performed the same multiple correlation analysis between prestimulus activity and movement time. As expected, we did not find any significant relationship in any of the investigated frequency bands, hence confirming that attention modulation selectively influenced reaction time.
Discussion
The main finding of this study is that, in patients with PD, the attentive processes promoting timely responses to unpredictable stimuli share neural resources with those underlying the learning of spatial sequences and working memory. Such common resources reside in frontoparietal network and involve frequencies mostly in the alpha band. In addition, this study expanded previous behavioral results in patients with PD (Marinelli et al. 2010) by showing that reaction time in a choice RT motor task is significantly correlated not only with the indices of declarative learning in a visuomotor sequence learning task, but also with the scores of short term and working memory in neuropsychological tests. Altogether, these findings suggest that, at least in PD, movement preparation, sequence learning and working memory abilities share common cognitive cores and neural resources.
In a previous work we have found that, in normal subjects, the modulation of brain rhythms in frontoparietal areas predicted the speed of visuomotor information processing in our choice RT motor task (Moisello et al. 2009b). The present results demonstrate, for the first time, that this is true also for patients with PD: subjects with less pronounced alpha and beta prestimulus desynchronization have longer reaction times. This relationship is particularly evident in the posterior electrode locations overlaying the parietal and occipital cortices for the low alpha frequency range. The new finding is that the performance in motor and non motor tasks of learning and working memory is significantly correlated with that same EEG pattern. From a behavioral point of view, the common denominator for reaction time, declarative sequence learning and working memory abilities appears to be attentive modulation, i.e., the ability to allocate attention and to bias adequate stimulus processing (Corbetta and Shulman 2002). Interestingly, one of the neurophysiological markers of attentive modulation has been identified in changes in EEG oscillations over parieto occipital areas before stimulus appearance (Klimesch et al. 1998; Romei et al. 2010 ; Sauseng et al. 2005; Thut et al. 2006; Zhang et al. 2008). Although novel, the results of this study are in line with those of perceptual and visuomotor investigations in normal subjects and animals (Hanslmayr et al. 2007; Zhang et al. 2008). Attentive modulation is likely the product of the interaction between frontal and parietal areas, more specifically, frontal eye field and intraparietal sulcus (Capotosto et al. 2009; Romei et al. 2010): frontoparietal areas may induce active engagement of posterior visual areas as seen by desynchronization of EEG activity. This EEG power change seems to be specific for the alpha range (Min and Herrmann 2007; Romei et al. 2010; Sauseng et al. 2005), although some studies have reported similar correlation between reaction time and beta activity (Hanslmayr et al. 2007; Zhang et al. 2008). Behaviorally, these processes translate into enhancement of attention, perception and action.
The results of this paper demonstrate that some motor and cognitive functions are not independent processes but share neural resources. Specifically, reaction time, a measure that have been considered “motor” in nature, has also a “cognitive” nature that is not shared by movement time. Reaction time in our task might reflect akinesia, while movement time might be an index of bradykinesia, two major motor signs of PD. Interestingly, we found that patients with longer reaction times (i.e., more akinetic) showed less desynchronization over the occipital areas, suggesting that attentional processes are less efficient in akinetic subjects. Indeed, we also found that these patients performed more poorly in the cognitive tasks of sequence learning and working memory. Thus, we hypothesize that impairment in the process of directing attention may be involved in the genesis of akinesia, as also suggested by the results of numerous behavioral studies in patients with PD (Berardelli et al. 2001; Jordan et al. 1992). On the other hand, movement time did not show any significant correlation either with the EEG activity or with the learning and working memory indices. These findings are important to characterize the signs of PD, as the UPDRS motor examination scores that are usually used in clinical settings do not provide a clear distinction between bradykinesia and akinesia, that have different response to pharmacological and other treatments (Berardelli et al. 2001).
Therefore, based on these findings, behavioral, pharmacological or interventional therapies could be designed to act specifically on attentional modulation and to improve, at the same time, motor and cognitive abilities. Moreover, the single measurement of reaction time in a choice RT task, such as the one used in these studies, could easily monitor therapy related attentional changes, overall if used in conjunction with EEG recording.
This hypothesis driven correlational approach that does not require a control population could be adopted not only in PD but also in many neurological disorders that involve frontoparietal dysfunction.
Acknowledgments
We thank Svetlana Kvint and April Pruski for assistance in data processing and Fiscellus Mons for the kind and precious support to the study. We also thank EET (Genova) for the software for behavioral data acquisition, analysis and EEG triggering. This work was supported by grants from: the McDonnell Foundation (GT, MFG), National Parkinson Foundation (MFG), NIH NS054864 (MFG), NIH NS055185 (GT), NIH P20MH077967 (GT).
References
- Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001;124:2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Reetz K, Ghilardi MF, Dhawan V, Eidelberg D. Early Parkinson's disease: longitudinal changes in brain activity during sequence learning. Neurobiol Dis. 2010;37:455–460. doi: 10.1016/j.nbd.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff GA. Principle component analysis of ERP data. In: Handy TC, editor. Event-Related Potentials A Methods Handbook. MIT Press; Cambridge, MA: 2005. pp. 189–207. [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008;21:478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Unified Parkinson's Disease Rating Scale. In: Recent developments in Parkinson's disease. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Mc Millian Healthcare Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- Folstein SE. Huntington's disease a disorder of families. Johns Hopkins University Press; Baltimore: 1989. [Google Scholar]
- Ghilardi MF, Eidelberg D, Silvestri G, Ghez C. The differential effect of PD and normal aging on early explicit sequence learning. Neurology. 2003;60:1313–1319. doi: 10.1212/01.wnl.0000059545.69089.ee. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Eidelberg D, Silvestri G, Ghez C. Abnormal spatial learning and motor planning in PD may share common bases. Mov Dis. 2004;19:S260. [Google Scholar]
- Ghilardi MF, Silvestri G, Feigin A, Mattis P, Zgaljardic D, Moisello C, Crupi D, Marinelli L, Dirocco A, Eidelberg D. Implicit and explicit aspects of sequence learning in pre-symptomatic Huntington's disease. Parkinsonism Relat Disord. 2008;14:457–464. doi: 10.1016/j.parkreldis.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. A comparison of clinical and pathological features of young- and old-onset Parkinson's disease. Neurology. 1988;38:1402–1406. doi: 10.1212/wnl.38.9.1402. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bauml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 2001;57:S11–26. [PubMed] [Google Scholar]
- Jordan N, Sagar HJ, Cooper JA. Cognitive components of reaction time in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 1992;55:658–664. doi: 10.1136/jnnp.55.8.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J. Induced alpha band power changes in the human EEG and attention. Neurosci Lett. 1998;244:73–76. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P, Gruber W. A short review of slow phase synchronization and memory: evidence for control processes in different memory systems? Brain Res. 2008;1235:31–44. doi: 10.1016/j.brainres.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Marinelli L, Perfetti B, Moisello C, Di Rocco A, Eidelberg D, Abbruzzese G, Ghilardi MF. Increased reaction time predicts visual learning deficits in Parkinson's disease. Mov Disord. 2010 doi: 10.1002/mds.23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min BK, Herrmann CS. Prestimulus EEG alpha activity reflects prestimulus top-down processing. Neurosci Lett. 2007;422:131–135. doi: 10.1016/j.neulet.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Moisello C, Crupi D, Tunik E, Quartarone A, Bove M, Tononi G, Ghilardi MF. The serial reaction time task revisited: a study on motor sequence learning with an arm-reaching task. Exp Brain Res. 2009a;194:143–155. doi: 10.1007/s00221-008-1681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisello C, Perfetti B, Landsness E, Naro A, Sarasso S, Onofrj M, Tononi G, Ghilardi MF. Society for Neuroscience. Chicago, IL: 2009b. Reaching and learning in choice-reaction time tasks: The differential topographical and temporal patterns of alpha and beta rhythms. [Google Scholar]
- Romei V, Gross J, Thut G. On the Role of Prestimulus Alpha Rhythms over Occipito-Parietal Areas in Visual Input Regulation: Correlation or Causation? J Neurosci. 2010;30:8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. The European journal of neuroscience. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – third revision (WAIS-III) The Psychological Corporation; 1997. [Google Scholar]
- Zhang Y, Wang X, Bressler SL, Chen Y, Ding M. Prestimulus cortical activity is correlated with speed of visuomotor processing. J Cogn Neurosci. 2008;20:1915–1925. doi: 10.1162/jocn.2008.20132. [DOI] [PubMed] [Google Scholar]