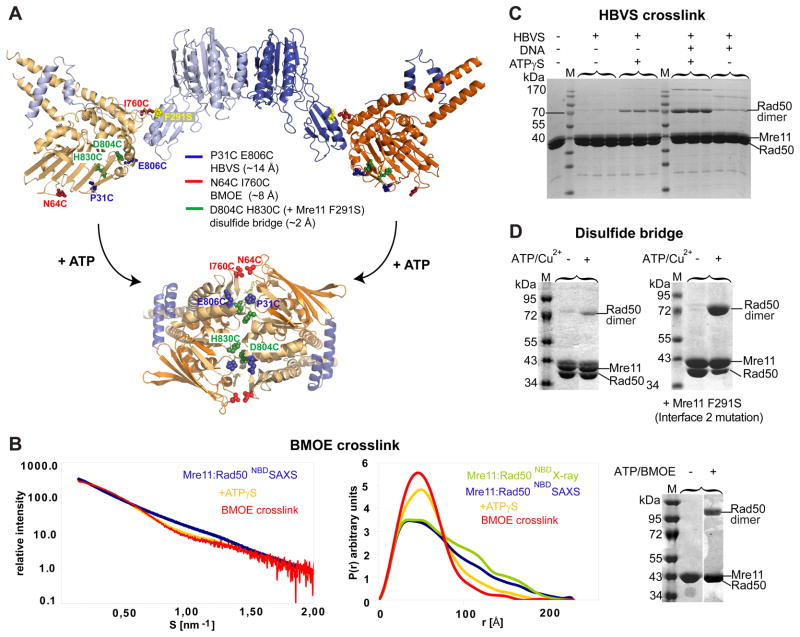
Figure 3. ATP engages Rad50NBDs in the catalytic head.
A) Cysteine mutations introduced in TmRad50NBD to test formation of the ATP bound Rad50 dimer. Sites are widely separated in the open form (upper crystal structure), but closely spaced for crosslinking or disulfide bonding in the ATP bound form (below, see Fig. 4).
B) Left panel: Superposition of experimental SAXS curves of Mre11:Rad50NBD with and without ATPγS indicate a more compact, globular shape in the presence of ATPγS. SAXS of the BMOE crosslinked Mre11:Rad50NBD,N64C,I760C results in a shape similar to the ATPγS bound form. Middle panel: the electron pair distance distribution function P(r) in the absence of nucleotides corresponds well to the crystal structure derived P(r). ATPγS increases the short distances and decreases the long distances. Residual long distances suggest a heterogeneous mixture between the open form and closed ATPγS complex. The BMOE crosslinked complex has a similar shape to the ATPγS complex, but appears to be more homogenous for the compact form. Right panel: Non-reducing Coomassie stained SDS PAGE of the MRNBD,N64C,I760C crosslinking experiment using BMOE. The crosslinking forms a covalently connected Rad50 dimer in an ATP dependent manner.
C) Chemical crosslinking by HBVS of MRNBD,P31C,E806C creates a covalently connected Rad50 dimer in an ATPγS and DNA dependent manner. The identity of the corresponding gel band was confirmed by mass spectrometry.
D) The formation of ATP bound engaged Rad50NBD,D804C,H830C is tested by using ATP/Cu2+ dependent disulfide bond formation. Modulating the Mre11:Rad50 interface 2 by Mre11F291S results in dramatically increased disulfide bond formation efficiency, consistent with the idea that interface 2 stabilizes the open form and is disrupted in the closed form.
See also Figure S3.