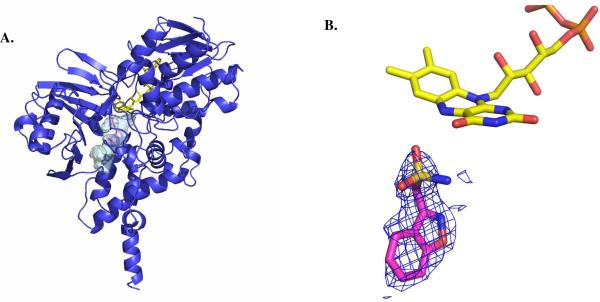
Figure 2.
Structural properties of Zonisamide binding to human MAO B. (A) Ribbon diagram of the overall structure of human MAO B in complex with zonisamide. The substrate-binding cavity is represented as semi-transparent surface. Zonisamide is shown in magenta, FAD in yellow, and the MAO B chain trace in blue. (B) Weighted 2Fo-Fc electron density of bound zonisamide in the active site of MAO B. The map was calculated before inclusion in the model of the inhibitor and, therefore, it is fully unbiased. Zonisamide carbons are in magenta, flavin carbons in yellow, oxygens in red, nitrogens in blue, sulphurs in light brown, and phosphorous in orange. This figure as well as Figures 3 and 4 were produced with PyMol (www.pymol.org).