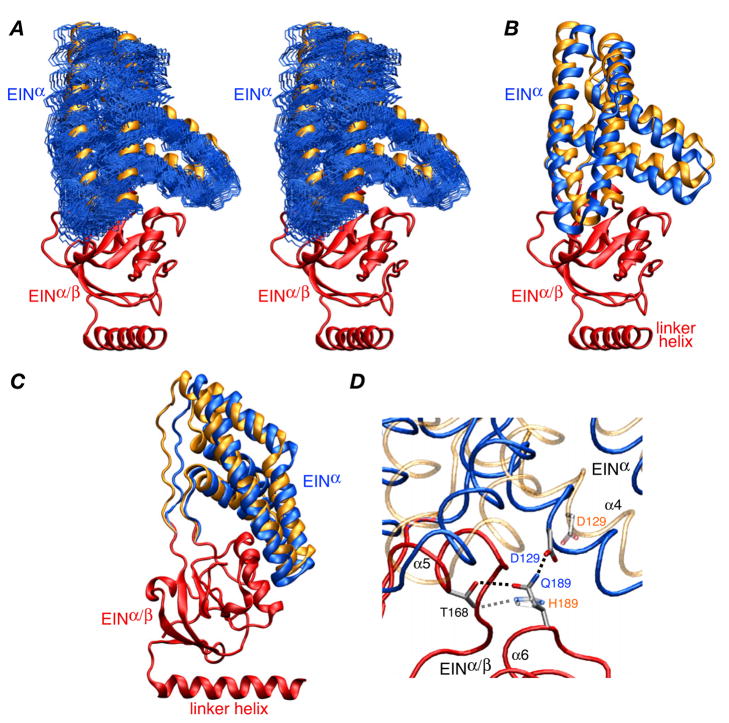
Figure 3.
Structure of the EIN domain in the EI(H189Q) dimer. (A) Stereoview of the final 100 simulated annealing structures (calculated with random noised added to the RDCs) best-fitted to the EINα/β subdomain (red ribbon) showing the precision with which the orientation of the EINα subdomain (backbone atoms in blue) relative to the EINα/β subdomain has been determined. The location of the EINα subdomain in wild-type EI9 is shown as a gold ribbon. (B) and (C) Two views of the EIN domain with the EINα/β subdomain superimposed (red) and the EINα subdomains of the restrained regularized mean structures of the EI(H189Q) mutant and wild-type EI9 in blue and gold, respectively. (D) Detail depicting the EINα/EINα/β interface and the hydrogen bonding interactions involving residues 189 (Gln in the mutant and His in the wild-type). The backbone of the EINα/β subdomain of the mutant and wild-type is superimposed (red tube), and the backbone of the EINα subdomain is shown as blue and transparent gold tubes for the mutant and wild-type, respectively. The side chains of Thr168, His189/Gln189 and Asp129 are displayed, with the mutant opaque and the wild-type transparent; the dashed lines indicate hydrogen bonding interactions.