Table 1.
Ligand Optimization for Phosphoramidite AuI-catalyzed [2+2]-Cycloaddition of 1b.a
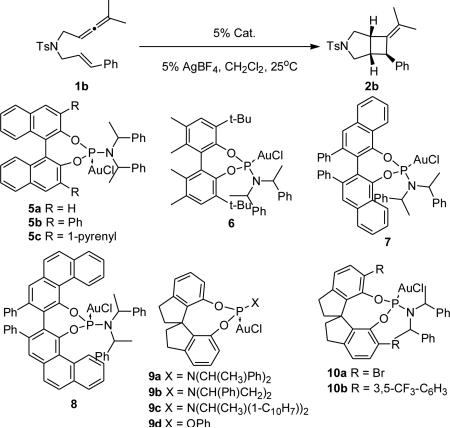 | |||
|---|---|---|---|
| entry | Cat. | yield (%)b | ee (%)c |
| 1 | (S,S,S)-5a | 81 | 34(+) |
| 2 | (S,S,S)-5b | 91 | 14(+) |
| 3 | (S,S,S)-5c | 67 | 56(+) |
| 4 | (S,S,S)-6 | 34 | 60(+) |
| 5 | (S,S,S)-7 | 81 | 70(+) |
| 6 | (R,R,R)-8 | 77 | 64(–) |
| 7 | (R,R,R)-9a | 86 | 90(+)d |
| 8 | (S,R,R)-9a | 73 | 52(–) |
| 9 | (R,R,R)-9b | 87 | 32(+) |
| 10 | (R,R,R)-9c | 22 | 4(+) |
| 11 | (R)-9d | 88 | 32(+) |
| 12 | (R,R,R)-10a | 66 | 56(–) |
| 13 | (R,R,R)-10b | --- | --- |
Reaction conditions: Cat (5 mol%), AgBF4 (5 mol%), dichloromethane, 25°C, 24h. Only one diastereoisomer was observed in all cases.
Isolated yields after silica gel flash column chromatography.
Enantiomeric excess determined by enantiodiscriminating HPLC (see Supporting Information).
The enantioselectivity was increased to 99% ee after a simple crystallization.
