Table 2.
Ligand Optimization for Phosphoramidite AuI-catalyzed [2+2]-Cycloaddition of 1 with (R,R,R)-9a.a
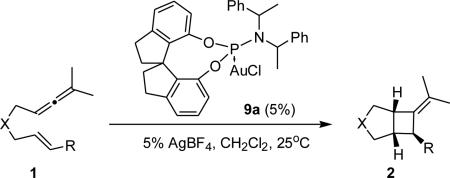 | |||||
|---|---|---|---|---|---|
| entry | 2 | X | R | yield (%)b | ee (%)c |
| 1 | a | C(CO2Me)2 | Ph | 75 | 14 |
| 2 | b | N-Ts | Ph | 86 | 94 |
| 3 | c | C(SO2Ph)2 | Ph | 82 | 85 |
| 4 | d | N-Boc | Ph | 52 | 81 |
| 5 | e | N-Ts | 2-OMe-C6H4 | 82 | 80 |
| 6 | f | N-Ts | 3-OMe-C6H4 | 80 | 91 |
| 7 | g | N-Ts | 4-OMe-C6H4 | 72 | 80 |
| 8 | h | N-Ts | 4-Cl-C6H4 | 87 | 97 |
| 9d | b | N-Ts | Ph | 67 | 90 |
Reaction conditions: Cat (5 mol%), AgBF4 (5 mol%), dichloromethane, 25°C, 24h. Only one diastereoisomer was observed in all cases. Trans-2 was used in all cases except for those noted.
Isolated yields after silica gel flash column chromatography.
Enantiomeric excess determined by enantiodiscriminating HPLC (see Supporting Information).
From cis-1b.
