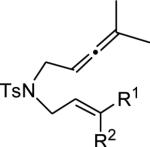
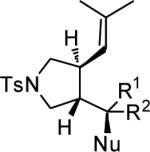
Table 4.
Substrate Scope for AuI-catalyzed Cycloaddition/Alkoxylation of Allenenes with 11c.a
| entry | Substrate | NuH | 3 | yield % | ee % |
|---|---|---|---|---|---|
|
|
||||
| R1, R2 | |||||
| 1 | 2-OMe-C6H4, H | MeOH | d | 73 | 96 |
| 2 | 3-OMe-C6H4, H | MeOH | e | 92 | 96 |
| 3 | 4-OMe-C6H4, H | MeOH | f | 90 | 94 |
| 4 | 4-Cl-C6H4, H | MeOH | g | 68 | 86 |
| 5 | Ph, H | EtOH | h | 90 | 92 |
| 6 | Ph, H | HOCH2(C(CH3)3) | i | 72 | 94 |
| 7 | Ph, H | HOCH2(HC=CH2) | j | 84 | 94 |
| 8 | Ph, H | HOCH(CH3)2 | k | 81 | 96 |
| 9 | Ph, H | H2O | l | 85 | 96 |
| 10 | Ph, H | MeOH | b | 95 | 94 |
| 11 | H, Ph | MeOH | b | 39 | 86 |
| 12 | Ph, Me | MeOH | m | 83 | 92 |
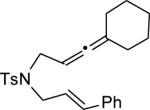
|
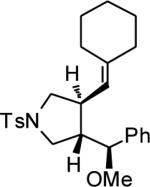
|
||||
| 13 | MeOH | n | 76 | 94 | |
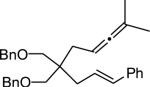
|
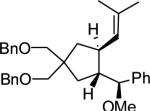
|
||||
| 14 | MeOH | o | 86 | 72 |
Reaction conditions: 11c (6 mol%), AgBF4 (5 mol%), trapping agent (Nu) (9 equiv), 24h. For entries 5-10 the reaction was run at 0°C, all others were run at room temperature. In all experiments a small amount (<9%) of the corresponding [2+2]-cycloaddition product was also produced. The diastereoselectivity was >98:2 in all cases. Isolated yields after silica gel flash column chromatography. Enantiomeric excess determined by enantiodiscriminating HPLC (see Supporting Information).
