Abstract
Aims/hypothesis
Homozygosity for glycine at codon 16 (GlyGly) of the β2-adrenergic receptor may alter receptor sensitivity upon chronic stimulation and has been implicated in the pathogenesis of hypoglycaemia unawareness. We compared the effect of antecedent hypoglycaemia on β2-adrenergic receptor sensitivity between GlyGly participants and those with arginine 16 homozygosity (ArgArg) for the β2-adrenergic receptor.
Methods
We enrolled 16 healthy participants, who were either GlyGly (n = 8) or ArgArg (n = 8). They participated randomly in two 2 day experiments. Day 1 consisted of two 2-h hyperinsulinaemic hypoglycaemic (2.8 mmol/l) or euglycaemic (4.8 mmol/l) glucose clamps. On day 2, we measured the forearm vasodilator response to the β2-adrenergic receptor agonist salbutamol and the dose of isoprenaline required to increase the heart rate by 25 bpm (IC25).
Results
The vasodilator response to salbutamol tended to be greater after antecedent hypoglycaemia than after euglycaemia (p = 0.078), consistent with increased β2-adrenergic receptor sensitivity. This effect was driven by a significant increase in β2-adrenergic receptor sensitivity following hypoglycaemia compared with euglycaemia in ArgArg participants (p = 0.019), whereas no such effect was observed in the GlyGly participants. Antecedent hypoglycaemia tended to decrease the IC25 in ArgArg participants, whereas the reverse occurred in the GlyGly participants (GlyGly vs ArgArg group p = 0.047).
Conclusion/interpretation
Antecedent hypoglycaemia did not affect β2-adrenergic receptor sensitivity in healthy GlyGly participants, but increased it in ArgArg participants. If these results also hold for participants with type 1 diabetes, such an increase in β2-adrenergic receptor sensitivity may potentially reduce the risk of repeated hypoglycaemia and the subsequent development of hypoglycaemia unawareness in ArgArg diabetic participants.
Trial registration
ClinicalTrials.gov NCT00160056
Funding
Radboud University Nijmegen Medical Centre.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-011-2062-3) contains supplementary material, which is available to authorised users.
Keywords: Adrenergic beta-2, Hypoglycaemia, Genome, Polymorphism single nucleotide, Receptors, Adrenergic, Type 1 diabetes
Introduction
Despite important advances in insulin treatment and glucose control, hypoglycaemia remains a fact of life for many patients with type 1 diabetes. Repeated hypoglycaemic episodes are known to impair glucose counter-regulatory defences and to reduce hypoglycaemic awareness [1–3]. Indeed, impairments in counterregulatory hormone responses to, and symptomatic perception of, insulin-induced hypoglycaemia can be induced by as few as two hypoglycaemic episodes, even in healthy participants [4–6]. However, although most patients with type 1 diabetes experience hypoglycaemia on a fairly regular basis, clinically relevant hypoglycaemia unawareness affects ‘only’ about 25% of patients [7]. This suggests involvement of other factors that determine the susceptibility to developing hypoglycaemia unawareness.
Genetic factors have been implicated in the risk for hypoglycaemia and hypoglycaemia unawareness in patients with type 1 diabetes [8, 9]. We recently found that the prevalence of hypoglycaemia unawareness was in part determined by a SNP in the gene encoding the β2-adrenergic receptor (ADRB2) [10]. Thus, patients who were GlyGly at codon 16 of the β2-adrenergic receptor were more likely to report hypoglycaemia unawareness than those who were ArgGly or ArgArg.
The SNP occurring at position 46 of ADRB2, encoding for arginine or glycine at the N-terminus (codon 16) of the β2-adrenergic receptor, may determine the degree to which β2-adrenergic receptors lose sensitivity when chronically stimulated. Although in vitro studies have displayed discrepant results [11], most in vivo data indicate that substitution of glycine for arginine at codon 16 reduces sensitivity of the β2-adrenergic receptor upon chronic stimulation [12, 13]. This might be a potential mechanism in the development of hypoglycaemia unawareness since recurrent hypoglycaemic episodes and the consequent release of catecholamine’s may alter β2-adrenergic receptor sensitivity. Reduced β-adrenergic sensitivity has been reported in relation to hypoglycaemia unawareness, based on reduced heart rate responses to isoprenaline, a non-selective β-adrenergic agonist [14–18]. In addition, there is some suggestion that this reduced sensitivity was mediated through the β2-adrenergic receptor [19, 20]. Mixed effects were seen in healthy participants, with one study showing reduced [21], and another showing increased, β-adrenergic sensitivity [15] after a period of hypoglycaemia. The effect of antecedent hypoglycaemia on β2-adrenergic sensitivity alone has not been determined. Further, it could be argued that genetic variation of the β2-adrenergic receptor explains the abovementioned disparate effects of hypoglycaemia on β-adrenergic sensitivity in patients and healthy participants.
The aims of this study were first to investigate whether two episodes of antecedent hypoglycaemia, a stimulus known to induce hypoglycaemia unawareness [4, 5], would decrease next day β2-adrenergic sensitivity in healthy participants, and second, to investigate this more particularly in participants who were GlyGly compared with ArgArg.
Methods
Participants
Healthy participants (n = 96) were selected by advertisement and genotyped for the Arg/Gly polymorphism in ADRB2. Of these, 16 participants were enrolled in the present study: eight GlyGly (one male, mean age 22 ± 1 years, mean BMI 21.2 ± 1.3 kg/m2) and eight ArgArg (four male, mean age 22 ± 1 years, mean BMI 21.2 ± 0.4 kg/m2). None of the participants were allowed to use any medication except oral contraceptives. They were asked to abstain from alcohol and caffeine containing products for 24 h and from food intake at least 10 h before experiments took place. All participants gave their written informed consent. The study was approved by the Radboud University Nijmegen Medical Centre Medical Ethics Committee and carried out in accordance with the principles of the Declaration of Helsinki as revised in 2000.
Experimental design
We performed a randomised controlled crossover study, comparing the effects of antecedent hypoglycaemia with those of antecedent euglycaemia. Participants participated in two experiments, each consisting of two consecutive days and scheduled at least 3 weeks apart. Female participants were studied during the same period of the menstrual cycle. The aim of day 1 was to induce hypoglycaemia unawareness by two consecutive episodes of hypoglycaemia, whereas the control experiment consisted of two comparable episodes of euglycaemia. The aim of day 2 was to quantify β2-adrenergic and overall β-adrenergic sensitivity by measuring the vasodilator response to salbutamol and the heart rate response to isoprenaline, respectively. All experiments took place at the Clinical Research Centre Nijmegen (CRCN) in a temperature controlled room (temperature 23–24°C).
First experiment day 1
All participants were admitted to the CRCN at 08:00 hours after an overnight fast. Two indwelling catheters were inserted intravenously. One catheter was placed in retrograde fashion into a dorsal hand vein for blood sampling. This hand was placed in a heated box (55–60°C) to obtain arterialised venous blood [22]. The second catheter was inserted in the antecubital vein of the contralateral arm for infusion of insulin (Actrapid; Novo Nordisk, Bagsvaerd, Denmark) and glucose. At t = 0 min, infusion of insulin was initiated at a rate of 60 mU m−2 min−1 after a bolus of 1 U. Plasma glucose was maintained at predetermined levels using a variable infusion of glucose 20%, based on plasma glucose levels measured in duplicate at 5 min intervals by the glucose oxidase method using a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA). The plasma glucose target was ~4.8 mmol/l during the euglycaemic clamps and ~2.8 mmol/l during the hypoglycaemic clamps. These levels were reached within 45 min and then maintained for 120 min. Subsequently, the insulin infusion was discontinued, euglycaemia was maintained (euglycaemia) or restored (hypoglycaemia), and the participants received a small snack. After 120 min, the clamp procedure was repeated in the afternoon. At t = 450 min, the insulin infusion was terminated, glucose was continued as required to restore and maintain euglycaemia and participants received a carbohydrate-rich meal before they left the research unit at approximately 18:00 hours. Arterialised blood was sampled for measurement of catecholamines, insulin, glucagon and cortisol at baseline, and at t = 45, 105, 165, 330, 390 and 450 min. Before initiation of, and at 20-min intervals during the clamp procedures, the appearance of hypoglycaemic symptoms was checked using a semi-quantitative symptom questionnaire [23] consisting of non-specific (not feeling well, nausea, headache), adrenergic (tremor, palpitations), cholinergic (hunger, sweating, dry mouth, tingling), neuroglycopenic (difficulty speaking, blurred vision, difficulty to concentrate, confusion, tiredness, weakness) and dummy symptoms (pain in legs, yellow vision). Participants were asked to score these items from 0 (absent) to 6 (very severe). In addition, they were also asked to score to what extent they felt hypoglycaemic.
First experiment day 2
Participants were re-admitted to the CRCN at 08:00 hours the next morning after an overnight fast. The brachial artery of the non-dominant arm was cannulated (Angiocath 20-gauche; Deseret Medical, Sandy, UT, USA) under local anaesthesia (xylocaine 2%) for infusion of salbutamol and continuous blood pressure monitoring. An indwelling catheter was inserted into the antecubal vein of the contralateral arm for infusion of isoprenaline. Intra-arterial infusion rates of salbutamol were calculated per 100 ml forearm volume, measured by water displacement. After cannulations, a 30-min equilibration period was allowed to pass before baseline variables were obtained. Subsequently, 5-min infusions of saline and incremental doses of salbutamol (Ventolin; GlaxoSmithKline, Zeist, the Netherlands) diluted in a saline vehicle (0.003, 0.01, 0.03, 0.1, 0.3, 1.0 μg/min per 100 ml of forearm tissue) were administered intra-arterially [24]. Forearm blood flow (FBF) was measured during the final 2 min of each dosing step in both arms, using ECG-triggered mercury-in-silastic strain gauge venous occlusion plethysmography as described previously [25]. Wrist cuffs inflated to 220 mmHg eliminated the hand circulation during FBF measurements [26]. The successive salbutamol doses were interrupted once by a 15-min drug-free interval for deflation of the wrist cuffs to allow recovery of hand circulation. The mean of eight FBF measurements was used for data analysis.
Thirty minutes after the FBF measurements, the isoprenaline sensitivity test was carried out [15, 27]. Participants were connected to a computer-assisted ECG (Fysioflex System, Nijmegen, the Netherlands) for determination of the heart rate response. Intra-arterial blood pressure was also recorded. Isoprenaline (Pharmacy department, University Medical Centre Groningen, the Netherlands) diluted in saline was injected intravenously as a 5 ml bolus infusion in incremental doses of 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0 and 4.0 μg or until the heart rate increased by >25 bpm. Each dose was given 2 min after the heart rate had returned to baseline level (approximately 5 min after the previous injection). The basal heart rate was defined as the mean heart rate for the 20 s before the first injection. The maximal heart rate was usually reached approximately 30–60 s following each injection. The maximal heart rate was determined as the mean of the three shortest consecutive RR intervals following each injection. β-Adrenergic sensitivity was expressed as the dose of isoprenaline that increased the heart rate by 25 bpm over baseline values (IC25) [15].
Analytical procedures
After genomic DNA isolation from blood [28], genotyping of the A/G (rs1042713) polymorphism in ADRB2 was performed by pyrosequencing [29] according to the manufacturer’s protocol (Pyrosequencing, Uppsala, Sweden). Further details are provided in the electronic supplementary material.
The total symptom score was calculated separately for each category at each time point. The means of the scores obtained during the morning and afternoon clamp were used for analysis. Blood samples for glucose measurements were centrifuged immediately. Arterialised blood for determination of catecholamines, insulin, glucagon and cortisol was kept on ice before centrifugation and then stored at −80°C for later analysis. Plasma adrenaline (epinephrine) and noradrenaline (norepinephrine) were analysed by HPLC with fluorometric detection, as described previously [30]. Plasma insulin was assessed by RIA using a human insulin standard (Novo Biolabs no 471), plasma glucagon was measured by competitive RIA using a reagent kit from Eurodiagnostica (Malmö, Sweden) and plasma cortisol was measured by Luminescence Immunoassay on an Architect random access analyser (Abbott, Hoofddorp, the Netherlands) [31]. The final two measurements during the morning clamps (i.e. t = 105 and t = 165 min) and the afternoon clamps (i.e. t = 390 and t = 450 min) were averaged for analysis.
Second experiment day 1 and day 2
With an interval of at least 3 weeks, all subjects returned for the second set of experiments. Participants who underwent a euglycaemic clamp on day 1 of the first experiment underwent a hypoglycaemic clamp on day 1 of the second experiment and vice versa. Procedures of days 1 and 2 were similar to those described above for the first experiment
Data analysis and statistical procedure
To detect differences in β2-adrenergic sensitivity between the hypoglycaemia and euglycaemia study arms (as measured by quantifying the forearm vasodilator response to salbutamol infusion), we calculated that a sample size of eight participants was required to reach a power of 80% to find a 25% difference at a two-sided significance level of 5%. Statistical analyses were performed using SPSS (software package 16.0). Differences in the means of continuous measurements were tested by two tailed Student’s t test. Vasodilator responses to salbutamol were expressed as absolute FBF. The effect of salbutamol on FBF was analysed by repeated-measures ANOVA. The IC25 was determined by calculating sigmoidal dose–response curves for each participant using Graphpad software (version 4.02). The IC25 levels following euglycaemia and hypoglycaemia were compared with paired or independent Student’s t tests as appropriate. p < 0.05 was considered statistically significant. Data are presented as means ± SEM.
Results
Insulin, glucose, glucose infusion rate and counterregulatory hormones during the clamps
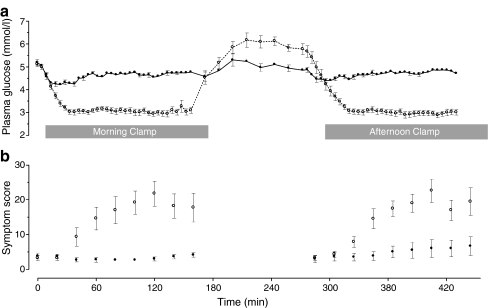
Mean plasma glucose levels were 4.9 ± 0.1 and 4.7 ± 0.0 mmol/l during morning and afternoon euglycaemia, respectively, vs 2.9 ± 0.1 and 2.9 ± 0.1 mmol/l during corresponding morning and afternoon hypoglycaemia (Fig. 1a). Insulin levels were similar during morning and afternoon euglycaemic and hypoglycaemic clamps (Table 1). Glucagon, adrenaline and cortisol responses were all significantly increased during both episodes of hypoglycaemia (Table 1). The glucagon response to afternoon hypoglycaemia was significantly attenuated compared with the response to morning hypoglycaemia. No significant differences in insulin levels, glucose levels, glucose infusion rate and counterregulatory hormone levels were observed between participants ArgArg and GlyGly or between males and females.
Fig. 1.
Plasma glucose values during the clamps on day 1 (a) and hypoglycaemic symptom scores during morning and afternoon clamps (b) in the whole group (n = 16). White circles, hypoglycaemia; black circles, euglycaemia
Table 1.
Plasma hormone and glucose levels and glucose infusion rate during morning and afternoon glucose clamps
| Measurement | Euglycaemia | Hypoglycaemia | ||
|---|---|---|---|---|
| Morning | Afternoon | Morning | Afternoon | |
| Glucose (mmol/l) | 4.9 (0.1) | 4.7 (0.1) | 2.9 (0.1)a | 2.9 (0.0)a |
| GIR (μmol kg-1 min-1) | 38.1 (1.1) | 43.8 (1.2) | 9.4 (1.7)a | 6.9 (1.6)a |
| Insulin (pmol/l) | 547 (28) | 580 (47) | 524 (40) | 564 (60) |
| Glucagon (pmol/l) | 22.4 (0.9) | 20.1 (0.6) | 47.4 (5.6)a | 32.8(3.5)ab |
| Cortisol (μmol/l) | 0.32 (0.03) | 0.27 (0.04) | 0.65 (0.04)a | 0.63 (0.05)a |
| Adrenaline (nmol/l) | 0.18 (0.02) | 0.20 (0.04) | 2.93 (0.40)a | 2.62 (0.27)a |
| Noradrenaline (nmol/l) | 1.37 (0.14) | 1.32 (0.15) | 1.64 (0.78) | 1.78 (0.19) |
Values are means (SEM)
GIR, glucose infusion rate
a p < 0.05 compared with euglycaemia; b p < 0.05 compared with morning experiment
Day 1 hypoglycaemic symptoms
Total hypoglycaemic symptom scores in the whole group were low during both morning and afternoon euglycaemia, and did not differ between ArgArg and GlyGly participants (Fig. 1b). During hypoglycaemia, scores were approximately sixfold higher, but did not differ between morning and afternoon hypoglycaemia, respectively, or between ArgArg and GlyGly participants. When adrenergic symptoms were analysed separately, there were no significant differences between morning and afternoon hypoglycaemia, or between ArgArg and GlyGly participants (data not shown).
Day 2 vasodilator response to salbutamol
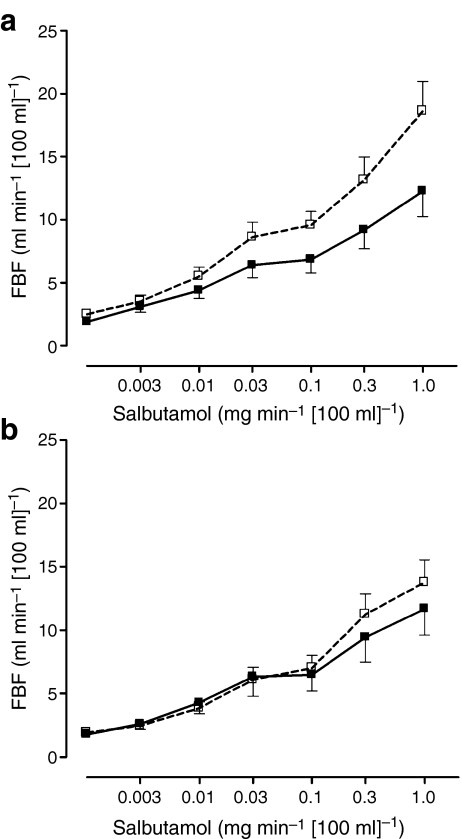
In response to salbutamol, FBF significantly increased following both euglycaemia (from 1.9 ± 0.15 ml min−1 [100 ml]−1 to 11.9 ± 1.4 ml min−1 [100 ml]−1) and hypoglycaemia (from 2.2 ± 0.22 ml min−1 [100 ml]−1 to 16.2 ± 1.5 ml min−1 [100 ml]−1) with the increase tending to be greater following hypoglycaemia (p = 0.078, ANOVA, data not shown). This would be consistent with increased β2-adrenergic receptor sensitivity following hypoglycaemia. This tendency towards increased β2-adrenergic receptor sensitivity was driven by a significant increase in β2-adrenergic sensitivity after antecedent hypoglycaemia in ArgArg participants, with a maximal response to salbutamol of 18.6 ± 2.3 ml min−1 [100 ml]−1 following hypoglycaemia and 12.2 ± 2.0 ml min−1 [100 ml]−1 following euglycaemia (p = 0.019, ANOVA, Fig. 2a), whereas no such effect was observed in GlyGly participants (Fig. 2b). Heart rate and mean arterial pressure remained unchanged until the final and highest dose of salbutamol was infused, when both increased, suggestive of systemic effects (data not shown).
Fig. 2.
Response of FBF to salbutamol following antecedent hypoglycaemia and antecedent euglycaemia in (a) ArgArg participants (n = 8, p = 0.019) and (b) GlyGly participants (n = 8, p = NS). White squares, hypoglycaemia; black squares, euglycaemia
Day 2 isoprenaline test
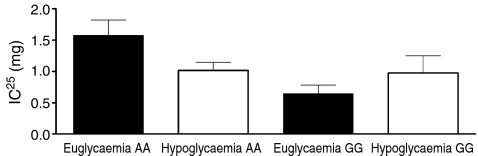
Two ArgArg and two GlyGly participants were excluded because of a technical error with the isoprenaline test or because the required heart rate response could not be reached or was already reached with the lowest dose of isoprenaline. In the remainder (n = 12), the IC25 measured after day 1 euglycaemia was higher in ArgArg participants than in GlyGly participants (1.57 ± 0.25 vs 0.65 ± 0.14 μg, p = 0.008). In response to two bouts of hypoglycaemia, the IC25 tended to decrease in ArgArg participants, suggestive of increased β-adrenergic sensitivity, whereas the opposite was seen in GlyGly participants. This diverging response to isoprenaline (the response following euglycaemia minus hypoglycaemia for both genotypes) following hypoglycaemia between the two subgroups was statistically significant (p = 0.047, Fig. 3).
Fig. 3.
IC25 following antecedent hypoglycaemia and antecedent euglycaemia in ArgArg participants (n = 6, p = 0.109) and GlyGly participants (n = 6, p = 0,193). The difference in IC25 between ArgArg and GlyGly participants (IC25 following antecedent euglycaemia minus antecedent hypoglycaemia for each subgroup): p = 0.047
Discussion
In the present study, two episodes of hypoglycaemia, a stimulus sufficient to impair next day counterregulatory responses and hypoglycaemic awareness in healthy participants [4, 5], did not decrease β2-adrenergic sensitivity in either participant subgroup. In contrast, β2-adrenergic sensitivity significantly increased following hypoglycaemia in participants who were homozygous ArgArg for the β2-adrenergic receptor, but did not change in those who were homozygous GlyGly. Analogously, hypoglycaemia induced an increase in overall β-adrenergic sensitivity measured with the isoprenaline test in ArgArg participants relative to the GlyGly participants. These findings suggest differences by genotype in the capacity to adapt to repeated hypoglycaemia.
Our finding of increased rather than reduced β2-adrenergic sensitivity after antecedent hypoglycaemia argues against a direct role for reduced β2-adrenergic sensitivity in the pathogenesis of hypoglycaemia unawareness. This seems at odds with studies reporting reduced overall β-adrenergic sensitivity in type 1 diabetic participants with hypoglycaemia unawareness [14, 16–18, 32] or healthy participants following hypoglycaemia [15, 21]. However, in contrast to these studies, we used a well-validated model for hypoglycaemia unawareness [5] and studied healthy participants rather than type 1 diabetic patients. In fact, the only other study that used a comparable approach reported that a single nocturnal hypoglycaemic episode increased next morning β-adrenergic sensitivity in healthy participants, whereas it decreased in patients with type 1 diabetes [15]. The authors interpreted this increase in β-adrenergic sensitivity as a compensatory response to prevent future hypoglycaemia, an adaptive process that was apparently lost or exhausted in patients with type 1 diabetes.
Our data raise the intriguing question as to whether the disparate responses to antecedent hypoglycaemia in the two genotype subgroups translate into a different susceptibility to developing hypoglycaemia unawareness in patients with type 1 diabetes. As mentioned above, one previous study has reported increased overall β-adrenergic sensitivity after hypoglycaemia in healthy non-diabetic participants [15]. Our findings are in accordance with these data insofar that the increase in β2-adrenergic sensitivity was limited to the ArgArg participants. This increase in β2-adrenergic sensitivity is potentially protective against future hypoglycaemic events. Conversely, GlyGly participants, who appear to lack this protective response, may be more prone to hypoglycaemic events and the subsequent development of hypoglycaemia unawareness. Although we realise that findings in healthy participants cannot be automatically translated to diabetic individuals, we speculate that this observation could potentially explain the association between hypoglycaemia unawareness and the GlyGly polymorphism for the β2-adrenergic receptor in type 1 diabetic patients [10].
Overall β-adrenergic sensitivity, following antecedent euglycaemia as reflected by the response to isoprenaline, appeared lower in ArgArg participants than in GlyGly participants, but tended to increase in ArgArg participants and to decrease in GlyGly participants following antecedent hypoglycaemia. However, although the effect of antecedent hypoglycaemia on overall β-adrenergic sensitivity in ArgArg participants was consistent with the effect on β2-adrenergic sensitivity, these data should be interpreted with caution. First, the time between the second hypoglycaemic episode on day 1 and the isoprenaline experiment on day 2 was at least 18 h, which is longer than in other studies where β-adrenergic sensitivity was measured either directly [21] or within ~10 h after the hypoglycaemic event [15]. Second, the isoprenaline test was performed shortly after β2-adrenergic sensitivity was assessed with salbutamol. Systemic effects of the final salbutamol dose could therefore have interfered with the subsequent isoprenaline test. Since β2-adrenergic sensitivity was the primary outcome, we deliberately chose to perform the isoprenaline test afterwards, whereas repeating the entire clamp procedure for this purpose was regarded as too great a burden for our participants. Third, since we had to exclude four participants from the analysis, we may have simply lacked the power to show meaningful effects. Finally, our observation that the differences in β-adrenergic sensitivity between the two genotype subgroups only applied to the euglycaemic experiments may indicate a baseline difference in adrenergic sensitivity or may reflect a general inaccuracy of the test method without clinical relevance.
One other limitation that deserves comment is that we did not formally test that hypoglycaemia unawareness was induced on day 2. However, others have repeatedly shown that two hypoglycaemic events are sufficient to impair counterregulatory hormone responses to and symptomatic awareness of next-day hypoglycaemia [4–6]. Furthermore, there was a reduced glucagon response compared with the preceding morning’s hypoglycaemia, indicating impending counterregulatory impairment.
In conclusion, antecedent hypoglycaemia increased β2-adrenergic sensitivity in young healthy participants ArgArg for ADRB2 in contrast to GlyGly participants who did not show increased β2-adrenergic sensitivity. We propose that non-diabetic participants ArgArg for ADRB2 may to some extent be protected against the development of some aspects of hypoglycaemia unawareness, but that GlyGly participants lack this protective mechanism. Further research, especially in diabetic participants, is necessary to explore this hypothesis. If this is confirmed in the type 1 diabetic population, it may help physicians to better anticipate the risk of developing hypoglycaemia unawareness when pursuing optimal glycaemic control.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 49 kb)
Acknowledgements
We thank A. Rennings, F. Poelkens, A. Jansen Van Rosendaal and J.-M. Kroese from the department of Internal Medicine for their help with performing the experiments and M. Coenen and B. Franke from the Department of Human Genetics for their help with genotyping the participants, all from the Radboud University Nijmegen Medical Centre. The results of this study were presented at the Scientific Meeting of the American Diabetes Association 2009 in New Orleans, LA, USA.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- ArgArg
Homozygous arginine
- ArgGly
Heterozygous arginine/glycine
- FBF
Forearm blood flow
- GlyGly
Homozygous glycine
- IC25
Isoprenaline concentration that increases the heart rate by 25 beats/min
- SNP
Single nucleotide polymorphism
References
- 1.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest. 1993;91:819–828. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45:937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 4.Davis MR, Shamoon H. Counterregulatory adaptation to recurrent hypoglycemia in normal humans. J Clin Endocrinol Metab. 1991;73:995–1001. doi: 10.1210/jcem-73-5-995. [DOI] [PubMed] [Google Scholar]
- 5.Davis SN, Mann S, Galassetti P, et al. Effects of differing durations of antecedent hypoglycemia on counterregulatory responses to subsequent hypoglycemia in normal humans. Diabetes. 2000;49:1897–1903. doi: 10.2337/diabetes.49.11.1897. [DOI] [PubMed] [Google Scholar]
- 6.Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes. 1991;40:223–226. doi: 10.2337/diabetes.40.2.223. [DOI] [PubMed] [Google Scholar]
- 7.Geddes J, Wright RJ, Zammitt NN, Deary IJ, Frier BM. An evaluation of methods of assessing impaired awareness of hypoglycemia in type 1 diabetes. Diab Care. 2007;30:1868–1870. doi: 10.2337/dc06-2556. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen-Bjergaard U, Agerholm-Larsen B, Pramming S, Hougaard P, Thorsteinsson B. Activity of angiotensin-converting enzyme and risk of severe hypoglycaemia in type 1 diabetes mellitus. Lancet. 2001;357:1248–1253. doi: 10.1016/S0140-6736(00)04405-6. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen-Bjergaard U, Dhamrait SS, Sethi AA, et al. Genetic variation and activity of the renin–angiotensin system and severe hypoglycemia in type 1 diabetes. Am J Med. 2008;121:246–248. doi: 10.1016/j.amjmed.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Schouwenberg BJ, Veldman BA, Spiering W, et al. The Arg16Gly variant of the beta2-adrenergic receptor predisposes to hypoglycemia unawareness in type 1 diabetes mellitus. Pharmacogenet Genomics. 2008;18:369–372. doi: 10.1097/FPC.0b013e3282f70481. [DOI] [PubMed] [Google Scholar]
- 11.Leineweber K, Brodde OE. Beta2-adrenoceptor polymorphisms: relation between in vitro and in vivo phenotypes. Life Sci. 2004;74:2803–2814. doi: 10.1016/j.lfs.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 13.Liggett SB. beta(2)-adrenergic receptor pharmacogenetics. Am J Respir Crit Care Med. 2000;161:S197–S201. doi: 10.1164/ajrccm.161.supplement_2.a1q4-10. [DOI] [PubMed] [Google Scholar]
- 14.Korytkowski MT, Mokan M, Veneman TF, Mitrakou A, Cryer PE, Gerich JE. Reduced beta-adrenergic sensitivity in patients with type 1 diabetes and hypoglycemia unawareness. Diab Care. 1998;21:1939–1943. doi: 10.2337/diacare.21.11.1939. [DOI] [PubMed] [Google Scholar]
- 15.Fritsche A, Stumvoll M, Grub M, et al. Effect of hypoglycemia on beta-adrenergic sensitivity in normal and type 1 diabetic subjects. Diab Care. 1998;21:1505–1510. doi: 10.2337/diacare.21.9.1505. [DOI] [PubMed] [Google Scholar]
- 16.Berlin I, Grimaldi A, Payan C, et al. Hypoglycemic symptoms and decreased beta-adrenergic sensitivity in insulin-dependent diabetic patients. Diab Care. 1987;10:742–747. doi: 10.2337/diacare.10.6.742. [DOI] [PubMed] [Google Scholar]
- 17.Berlin I, Grimaldi A, Bosquet F, Puech AJ. Decreased beta-adrenergic sensitivity in insulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1986;63:262–265. doi: 10.1210/jcem-63-1-262. [DOI] [PubMed] [Google Scholar]
- 18.Trovik TS, Jaeger R, Jorde R, Sager G. Reduced sensitivity to beta-adrenoceptor stimulation and blockade in insulin dependent diabetic patients with hypoglycaemia unawareness. Br J Clin Pharmacol. 1994;38:427–432. doi: 10.1111/j.1365-2125.1994.tb04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwab KO, Menche U, Schmeisl G, Lohse MJ. Hypoglycemia-dependent beta2-adrenoceptor downregulation: a contributing factor to hypoglycemia unawareness in patients with type-1 diabetes? Horm Res. 2004;62:137–141. doi: 10.1159/000080064. [DOI] [PubMed] [Google Scholar]
- 20.Trovik TS, Vaartun A, Jorde R, Sager G. Dysfunction in the beta 2-adrenergic signal pathway in patients with insulin dependent diabetes mellitus (IDDM) and unawareness of hypoglycaemia. Eur J Clin Pharmacol. 1995;48:327–332. doi: 10.1007/BF00194946. [DOI] [PubMed] [Google Scholar]
- 21.Trovik TS, Jaeger R, Jorde R, Sager G. Reduced beta-adrenergic sensitivity in healthy participants induced by hypoglycemia. Fundam Clin Pharmacol. 1995;9:181–186. doi: 10.1111/j.1472-8206.1995.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Moberg E, Kollind M, Lins PE, Adamson U, MacDonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia. 1992;35:287–290. doi: 10.1007/BF00400932. [DOI] [PubMed] [Google Scholar]
- 23.Deary IJ, Hepburn DA, MacLeod KM, Frier BM. Partitioning the symptoms of hypoglycaemia using multi-sample confirmatory factor analysis. Diabetologia. 1993;36:771–777. doi: 10.1007/BF00401150. [DOI] [PubMed] [Google Scholar]
- 24.de Galan BE, De Mol P, Wennekes L, Schouwenberg BJ, Smits P. Preserved sensitivity to beta2-adrenergic receptor agonists in patients with type 1 diabetes mellitus and hypoglycemia unawareness. J Clin Endocrinol Metab. 2006;91:2878–2881. doi: 10.1210/jc.2006-0528. [DOI] [PubMed] [Google Scholar]
- 25.Meijer P, Wouters CW, van den Broek PH, et al. Dipyridamole enhances ischaemia-induced reactive hyperaemia by increased adenosine receptor stimulation. Br J Pharmacol. 2008;153:1169–1176. doi: 10.1038/bjp.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenders J, Janssen GJ, Smits P, Thien T. Role of the wrist cuff in forearm plethysmography. Clin Sci (Lond) 1991;80:413–417. doi: 10.1042/cs0800413. [DOI] [PubMed] [Google Scholar]
- 27.Cleaveland CR, Rangno RE, Shand DG. A standardized isoproteranol sensitivity test. The effects of sinus arrhythmia, atropine, and propranolol. Arch Intern Med. 1972;130:47–52. doi: 10.1001/archinte.130.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronaghi M. Pyrosequencing for SNP genotyping. Methods Mol Biol. 2003;212:189–195. doi: 10.1385/1-59259-327-5:189. [DOI] [PubMed] [Google Scholar]
- 30.Willemsen JJ, Ross HA, Jacobs MC, et al. Highly sensitive and specific HPLC with fluorometric detection for determination of plasma epinephrine and norepinephrine applied to kinetic studies in humans. Clin Chem. 1995;41:1455–1460. [PubMed] [Google Scholar]
- 31.de Galan BE, Tack CJ, Lenders JW, et al. Theophylline improves hypoglycemia unawareness in type 1 diabetes. Diabetes. 2002;51:790–796. doi: 10.2337/diabetes.51.3.790. [DOI] [PubMed] [Google Scholar]
- 32.Berlin I, Grimaldi A, Landault C, et al. Lack of hypoglycemic symptoms and decreased beta-adrenergic sensitivity in insulin-dependent diabetic patients. J Clin Endocrinol Metab. 1988;66:273–278. doi: 10.1210/jcem-66-2-273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 49 kb)