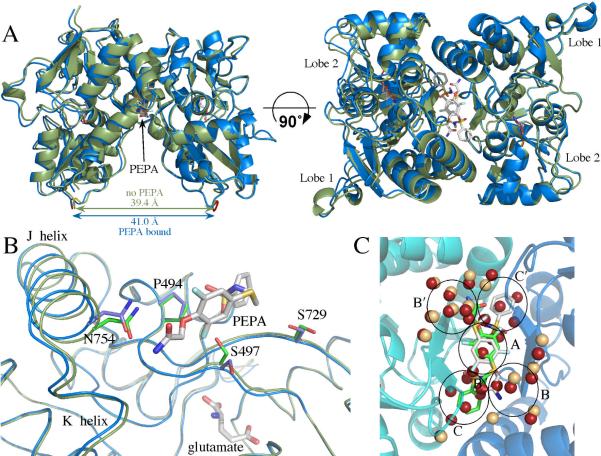
Figure 1.
(A) Comparison of glutamate-bound GluA2o S1S2 in the presence (blue) and the absence of PEPA (green) in two orientations. Both orientations of PEPA are shown. Note that the binding of PEPA results in a separation of the two components of the dimer (distance between the Cα atoms of the threonine in the linker) by approximately 1.5 Å. (B) One monomer of GluA2o S1S2 in the presence (blue) and the absence of PEPA (green) with one orientation of PEPA shown. Both the J/K helices and the strand near S497 are displaced upon binding PEPA. Also, the sidechains of S497 and S729 change rotameric states. (C) Comparison of the water molecules at the dimer interface in the presence (tan spheres) and the absence of PEPA (red spheres). PEPA is shown in both orientations. Despite the greater separation of the dimer interface, a number of the ordered water molecules found in the absence of PEPA are displaced by PEPA. The black circles delineate subsites of the allosteric modulator binding site as described previously (31).