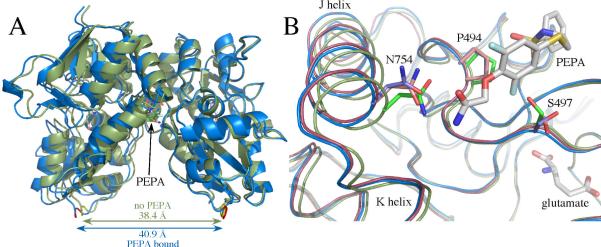
Figure 3.
(A) Comparison of glutamate-bound GluA3o S1S2 in the presence (blue) and the absence of PEPA (green) in two orientations. Both orientations of PEPA are shown. Note that the binding of PEPA results in a separation of the two components of the dimer (distance between the Cα atoms of the threonine in the linker) by approximately 2.5 Å. (B) One monomer of GluA3o S1S2 in the presence (blue) and the absence of PEPA (green) with one orientation of PEPA shown. Shown for comparison is the PEPA-bound form of GluA2o (red). Both the J/K helices and the strand near S497 are displaced upon binding PEPA for both GluA2o and GluA3o. Also, the sidechains of S497 and S729 are in different rotameric states for GluA3o bound to PEPA compared with GluA3o in the absence of PEPA and GluA2o bound to PEPA. Also, N754 is displaced in PEPA-bound GluA3o, such that only one H-bond is possible with the amide of PEPA.