Figure 5.
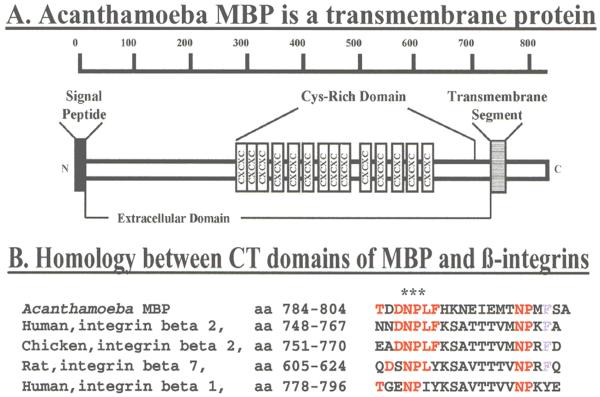
MBP cDNA codes for a precursor protein of 833 amino acids. Analysis of the deduced amino acid sequence using various programs revealed that residues 1-21 correspond to the MBP signal peptide and that amoeba MBP is a transmembrane protein (Panel A) containing a 712 amino acid long N-terminal extracellular (EC) domain (residues 22-733), a 22 amino acid long transmembrane domain (residues 734-755) and a short, 78 amino acid long, C-terminal cytoplasmic (CT) domain. The CT domain of MBP contains an NPLF motif that is identical to the one present in the cytoplasmic tail of chicken and human [beta]β2 integrins and is similar to the NPXY motif that is present in a number of other integrins (Panel B, red). The motifs NPLF/NPXY have been shown to bind to a number of intracellular proteins including talin, Dab, and tensin and play a key role in integrin activation and cell signaling events leading to stable cell adhesion, growth control and cell spreading. (Top panel reprinted with permission from Garate M et al.21)