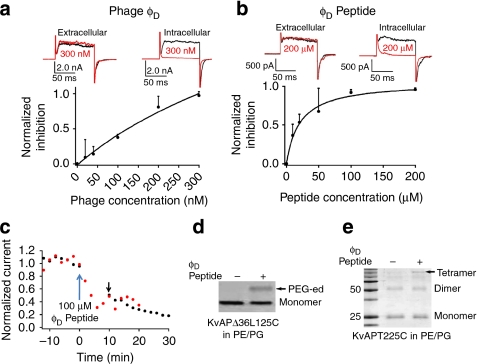
Figure 5. A 'down' conformation-specific ligand for KvAP in phospholipids.
(a) Phage φD inhibits channels in PE/PG. Top shows current traces with 300 nM phages on either side of the bilayer (black and red traces recorded before and 20 min after phage addition, respectively). Bottom is the dose-dependence for intracellular phage (black dots, s.d.; n=3). The continuous line is a polynomial fit (Supplementary Note S4). (b) Synthetic φD peptide (GNHAEIYSNHHMMSHVGGGR) inhibits KvAP. Top traces represent typical results for 200 μM peptide on either side of the bilayer (black and red traces recorded before and 20 min after peptide addition, respectively). The bottom shows the normalized inhibition (I) versus peptide concentration (black dots, s.d.; n=3; black line from a fitting with I=Imax/(1+kD/[peptide]), kD=21.2±3.5 μM). (c) φD Peptide binds to channels in resting state. Peptides at a concentration of 100 μM were added to the intracellular side at time 0. Two procedures were compared. One was that test pulses were delivered every 2 min (red dots). The other was that after the addition of the peptide, the membrane was held at −80 mV for 10 min before the delivery of next test pulse (black dots). The black arrow marks the first response after 10 min silence. (d) φD peptide makes KvAPΔ36L125C accessible in PE/PG. In the presence (+) or absence (+) of 100 μM peptide, channels were reduced, separated from reducing agents, incubated with 1.0 mM MTSPEG5k, quenched with 10 mM iodoacetamide and analysed in 15% nonreducing SDS–PAGE. (e) φD Peptide favoured the closed pore. In presence (+) or absence (−) of 100 μM peptide, full-length KvAP-T225C in PE/PG were reduced, separated from reducing agent and treated with 5.0 mM GSSG peptide for 2–3 min ('blank period') before nonreducing SDS––PAGE.