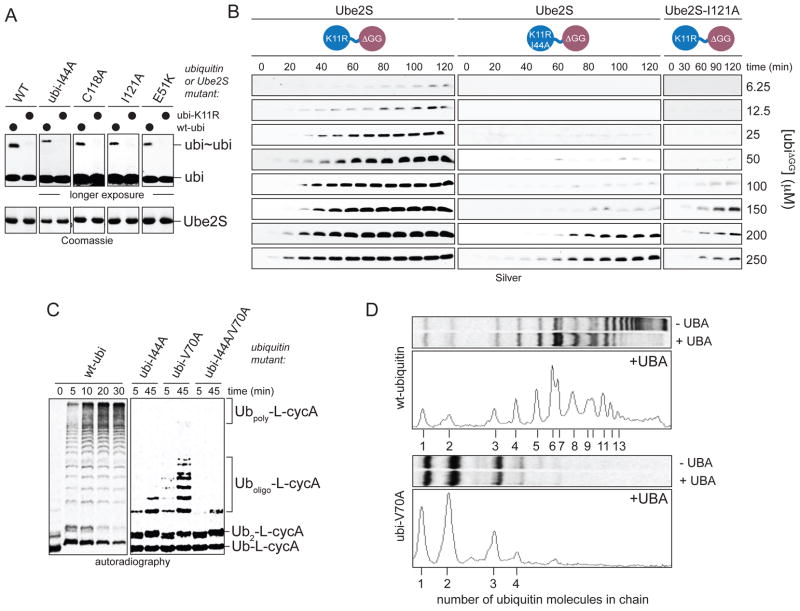
Figure 4. Non-covalent donor-binding increases the processivity of Ube2S.
A. Donor-binding is not required for the K11-linkage specificity of Ube2S. Ube2S or donor-binding mutants were incubated with ubiquitin or ubiK11R, or as indicated with ubiI44A and ubiI44A/K11R. Reactions were incubated longer and at higher ubiquitin concentrations to observe formation of ubi2 and analyzed by Coomassie staining. B. Donor-binding promotes catalysis at low acceptor concentrations. Dimer formation between increasing levels of ubiΔGG and ubiK11R or ubiK11R/I44A, respectively, by Ube2S was monitored by Silver staining. C. Time-course analysis of chain elongation on Ub-L-cycA by APC/CCdh1 and Ube2S in the presence of ubiquitin mutants, as analyzed by autoradiography. D. Donor binding is required for processive chain formation by Ube2S. Chain elongation on Ub-L-cycA by APC/C and Ube2S was monitored in the presence of the UBA-domains of Rad23A. Reactions were performed with ubiquitin or ubiV70A and analyzed by autoradiography (top) and line-scanning (bottom).