Abstract
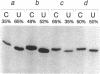
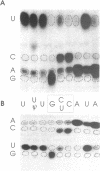
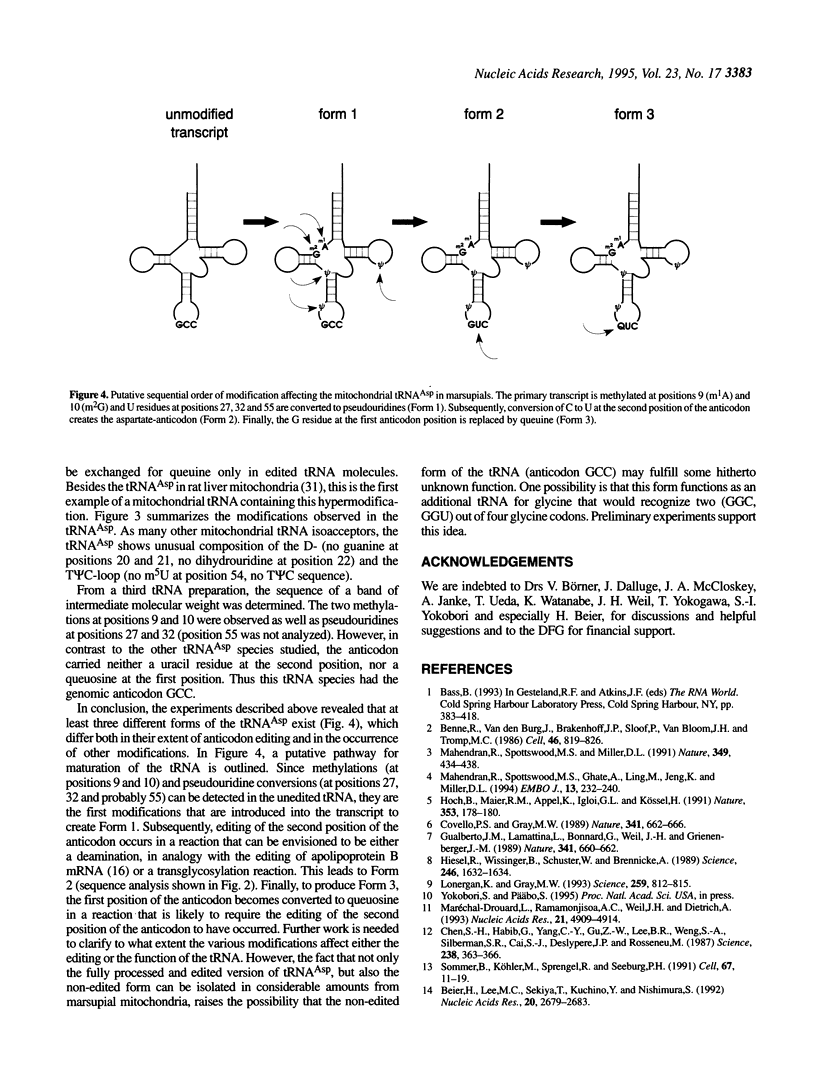
In marsupial mitochondria, the nucleotide residue at the second position of the anticodon of the tRNA for aspartic acid is changed post-transcriptionally such that the translational machinery recognizes it as a uracil rather than the cytosine residue encoded in the gene. By postlabeling nucleotide analysis, we show here that the cytosine residue is converted to a conventional uracil residue in an RNA editing event that affects approximately half of the tRNA molecules under steady state conditions. Furthermore, we have identified three different tRNA(Asp) species which all carry three pseudouridines and two methylations but have the anticodons GCC, GUC and QUC respectively, the latter representing a rare example of queuine incorporation into a mitochondrial tRNA. This allows us to describe a likely sequential order of modification of the tRNA(Asp), where methylations and conversions of uridines to pseudouridines precede the editing event, while the exchange of guanine by queuine takes place after the C to U editing event.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beier H., Lee M. C., Sekiya T., Kuchino Y., Nishimura S. Two nucleotides next to the anticodon of cytoplasmic rat tRNA(Asp) are likely generated by RNA editing. Nucleic Acids Res. 1992 Jun 11;20(11):2679–2683. doi: 10.1093/nar/20.11.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Van den Burg J., Brakenhoff J. P., Sloof P., Van Boom J. H., Tromp M. C. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986 Sep 12;46(6):819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- Carbon P., Haumont E., Fournier M., de Henau S., Grosjean H. Site-directed in vitro replacement of nucleosides in the anticodon loop of tRNA: application to the study of structural requirements for queuine insertase activity. EMBO J. 1983;2(7):1093–1097. doi: 10.1002/j.1460-2075.1983.tb01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Habib G., Yang C. Y., Gu Z. W., Lee B. R., Weng S. A., Silberman S. R., Cai S. J., Deslypere J. P., Rosseneu M. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987 Oct 16;238(4825):363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Covello P. S., Gray M. W. RNA editing in plant mitochondria. Nature. 1989 Oct 19;341(6243):662–666. doi: 10.1038/341662a0. [DOI] [PubMed] [Google Scholar]
- Gualberto J. M., Lamattina L., Bonnard G., Weil J. H., Grienenberger J. M. RNA editing in wheat mitochondria results in the conservation of protein sequences. Nature. 1989 Oct 19;341(6243):660–662. doi: 10.1038/341660a0. [DOI] [PubMed] [Google Scholar]
- Hiesel R., Wissinger B., Schuster W., Brennicke A. RNA editing in plant mitochondria. Science. 1989 Dec 22;246(4937):1632–1634. doi: 10.1126/science.2480644. [DOI] [PubMed] [Google Scholar]
- Hoch B., Maier R. M., Appel K., Igloi G. L., Kössel H. Editing of a chloroplast mRNA by creation of an initiation codon. Nature. 1991 Sep 12;353(6340):178–180. doi: 10.1038/353178a0. [DOI] [PubMed] [Google Scholar]
- Janke A., Päbo S. Editing of a tRNA anticodon in marsupial mitochondria changes its codon recognition. Nucleic Acids Res. 1993 Apr 11;21(7):1523–1525. doi: 10.1093/nar/21.7.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan K. M., Gray M. W. Editing of transfer RNAs in Acanthamoeba castellanii mitochondria. Science. 1993 Feb 5;259(5096):812–816. doi: 10.1126/science.8430334. [DOI] [PubMed] [Google Scholar]
- Mahendran R., Spottswood M. R., Miller D. L. RNA editing by cytidine insertion in mitochondria of Physarum polycephalum. Nature. 1991 Jan 31;349(6308):434–438. doi: 10.1038/349434a0. [DOI] [PubMed] [Google Scholar]
- Mahendran R., Spottswood M. S., Ghate A., Ling M. L., Jeng K., Miller D. L. Editing of the mitochondrial small subunit rRNA in Physarum polycephalum. EMBO J. 1994 Jan 1;13(1):232–240. doi: 10.1002/j.1460-2075.1994.tb06253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Ramamonjisoa D., Cosset A., Weil J. H., Dietrich A. Editing corrects mispairing in the acceptor stem of bean and potato mitochondrial phenylalanine transfer RNAs. Nucleic Acids Res. 1993 Oct 25;21(21):4909–4914. doi: 10.1093/nar/21.21.4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher T., Maas S., Higuchi M., Keller W., Seeburg P. H. Editing of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR-B pre-mRNA in vitro reveals site-selective adenosine to inosine conversion. J Biol Chem. 1995 Apr 14;270(15):8566–8570. doi: 10.1074/jbc.270.15.8566. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Yokoyama S., Horie N., Matsuda A., Ueda T., Yamaizumi Z., Kuchino Y., Nishimura S., Miyazawa T. A novel lysine-substituted nucleoside in the first position of the anticodon of minor isoleucine tRNA from Escherichia coli. J Biol Chem. 1988 Jul 5;263(19):9261–9267. doi: 10.1351/pac198961030573. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Agrawal H. P., Randerath E. tRNA alterations in cancer. Recent Results Cancer Res. 1983;84:103–120. doi: 10.1007/978-3-642-81947-6_7. [DOI] [PubMed] [Google Scholar]
- Rueter S. M., Burns C. M., Coode S. A., Mookherjee P., Emeson R. B. Glutamate receptor RNA editing in vitro by enzymatic conversion of adenosine to inosine. Science. 1995 Mar 10;267(5203):1491–1494. doi: 10.1126/science.7878468. [DOI] [PubMed] [Google Scholar]
- Sommer B., Köhler M., Sprengel R., Seeburg P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991 Oct 4;67(1):11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Steinberg S., Misch A., Sprinzl M. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1993 Jul 1;21(13):3011–3015. doi: 10.1093/nar/21.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. H., Sklar P., Axel R., Maniatis T. Editing of glutamate receptor subunit B pre-mRNA in vitro by site-specific deamination of adenosine. Nature. 1995 Mar 2;374(6517):77–81. doi: 10.1038/374077a0. [DOI] [PubMed] [Google Scholar]