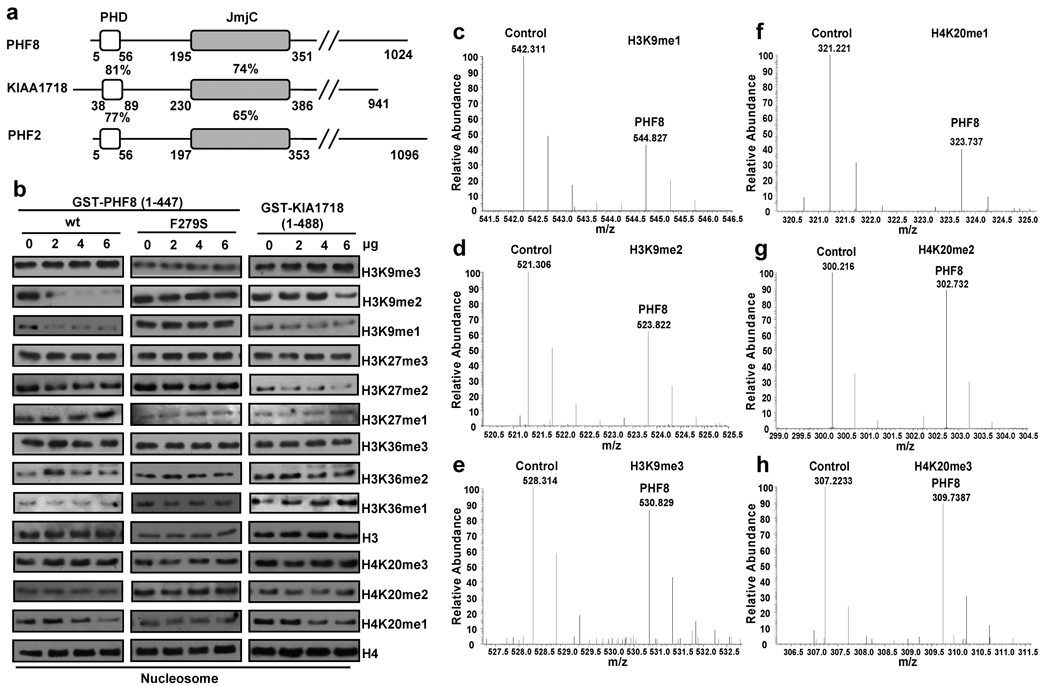
Figure 1. PHF8 and KIA1718 demethylate multiple lysines of histone 3 and 4 in vitro.
a. Schematic diagram of human PHF8 subfamily proteins. Percentages indicate identical amino acid in the PHD and JmjC domains. b. In vitro demethylation assays. Mono-nucleosomes (1µg) were incubated with GST-fused wtPHF8, PHF8 (F279S) and KIAA1718 proteins followed by Western blot with indicated antibodies. c–h. Mass spectrometry analysis of Control (GST) and PHF8 reacted nucleosomes, which were isotopically labeled with D0-propionyl and D5-propionyl, respectively. This labeling induces a 5 Da mass shift between the two samples, which is observed as a 2.5 m/z shift for doubly charged peptides. The m/z ranges depicting peptides are indicated. Decreases in H3K9me1 (c), H3K9me2 (d) and H4K20me1 (f) were detected from the PHF8 reaction compared to GST only. No changes were observed in others.