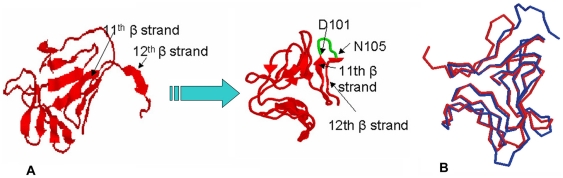
Figure 2. Comparison between the homology models of ASAL and mASAL.
(A) The homology model of ASAL to mASAL, showing that after incorporation of the loop between the 11th and 12th β-strand, the flanking C-terminal peptide folds back towards the central axis and thereby maintains the overall β-prism II fold. (B) Superimposition of mASAL (red) and the counterpart of ASAL (blue). The inflection point for the radical shift appears at position 98 (GNA numbering), from which point the C-terminal peptide moves in a completely different direction. In ASAL, the C-terminal peptide protrudes from the central axis of the molecule, whereas in mASAL, the 12th β-strand is folded to form a homogeneous β-sheet.