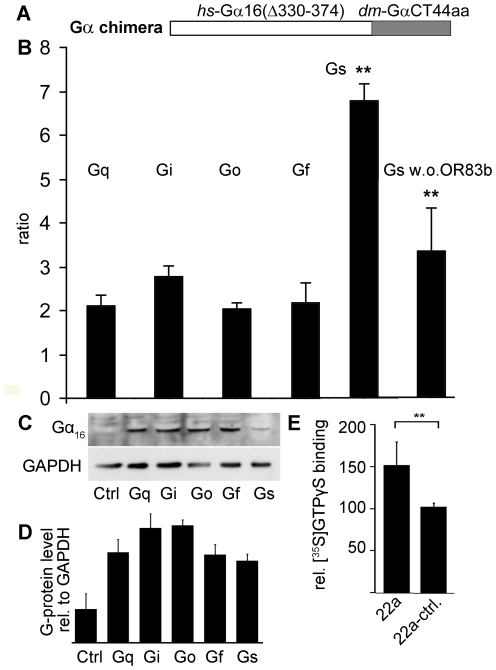
Figure 4. Role of G-proteins in signaling of recombinantly expressed olfactory receptors.
(A) Chimeric construct with N-terminus of human Gα16 and the C-terminus of Drosophila Gα-proteins. (B) Ratio of transfected HEK293 cells responding to 500 µM cyclohexanone in Ca2+ imaging experiments; cells either express OR43a, OR83b and the respective G-protein chimera, OR43a alone and the respective G-protein chimera, or OR43a, OR83b and full length human Gα16. An increase in the ratio means that more cells responded upon co-expression of the G-protein chimera (n>5 independent transfections). The ratio determined with the Gαs chimera was compared to the ratios obtained with the other G-protein chimera and was found to be signify-cantly different from all of them. (C) Western blot detection of recombinantly expressed G-protein chimera with an antibody against Gα16, equal amounts of protein was loaded per lane (controlled by GAPDH detection), control (ctrl) were non-transfected HEK293 cells. (D) Quantification of western blot analysis by densiometry, G-protein and GAPDH bands were ana-lyzed from cell preparations from 3 independent transfections. Band intensities originating from Gα16 stained membranes were divided by band intensities from GAPDH stained membranes. The small differences between the intensities from the different chimera were not significant (tested with Student's t-test). (E) [35S]GTPγS binding to membrane preparations upon odorant stimu-lation, cells were transfected with ORs and Gα-protein, control cells were mock transfected. The reaction was carried out in triplicates. Differences between the indicated data points were statistically checked by the unpaired Student's t test, **p<0.01. Error bars represent s.e.m.