Fig 4.
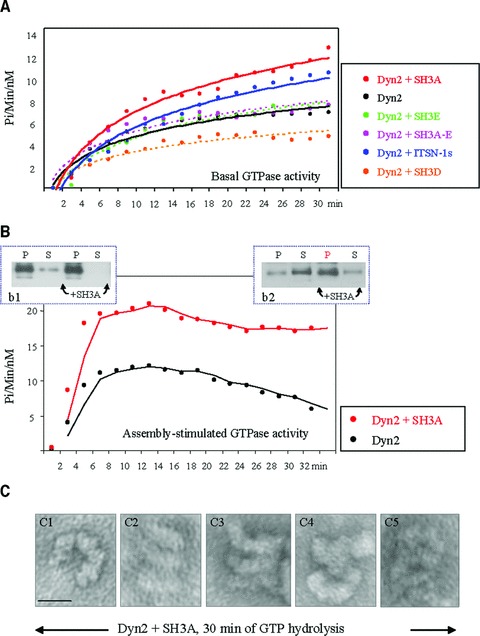
GST-SH3A stimulates the GTPase activity of dyn2 and stabilizes dyn2 oligomeric structures. (A) The basal GTPase activity of dyn2 measured in the presence of different GST-SH3 domains of ITSN-1s as well as full length GST-tagged ITSN-1s. (B) Oligomeric dyn2 structures (b1, P fractions) were subjected to GTPase assay in the absence or presence of the GST-SH3A of ITSN-1. The GTPase activity of assembled dyn2 in the presence of GST-SH3A is higher by comparison to dyn2 alone. The GTPase activity of dyn2 decreases as GTP is hydrolysed and the dyn2 rings disassemble. In the presence of GST-SH3A, despite GTP hydrolysis, the GTPase activity of dyn2 remains high and apparently dyn2 conformation does not change. As shown, after 30 min. of GTP hydrolysis, the amount of pelletable dyn2 is significantly high (b2). (C) EM– staining of stabilized dyn2 oligomeric structures, 30 min. after GTP hydrolysis. Dyn2 oligomeric structures – open rings (c1), stacks of rings (c2–c5), are stabilized, apparently unable to change their conformation. Bar: 50 nm.
