Table 3.
Effects of hydrophobic domain on IN inhibition
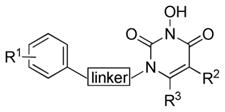 | ||||||
|---|---|---|---|---|---|---|
| Compd | R1 | linker | R2 | R3 | IN IC50 (μM)a |
|
| 3′P | STb | |||||
| 11c | H | CH2OCH2 | iPr | benzyl | >111 | 21 ± 2 |
| 51 | H | CH2OCH2 | iPr | 4-Fbenzyl | >111 | 7.3 ± 0.8 |
| 52 | F | CH2OCH2 | iPr | 4-Fbenzyl | >111 | 5.5 ± 0.7 |
| 53 | H | CH2OCH2 | Et | benzyl | >111 | 8.2 ± 1.0 |
| 54 | H | CH2OCH2 | Me | H | >111 | 75 ± 7 |
| 55 | H | CH2OCH2 | benzyl | Et | >111 | 36 ± 6 |
| 56 | F | CH2OCH2 | benzyl | Et | >111 | 11 ± 0.8 |
| 64 | H | CH2 | iPr | 4-Fbenzyl | >111 | 32 ± 6 |
| 65 | F | CH2 | iPr | 4-Fbenzyl | >111 | 73 ± 12 |
| 66 | H | CH2 | iPr | benzyl | >111 | 28 ± 2 |
| 67 | H | CH2 | Et | benzyl | >111 | >111 |
| 68 | F | CH2 | Et | benzyl | >111 | 24 ± 3 |
| 69 | H | CH2 | Me | H | >111 | >111 |
| 70 | F | CH2 | Me | H | >111 | >111 |
| 75 | H | CH2 | benzo (fused) | >111 | >111 | |
| 76 | F | CH2 | benzo (fused) | >111 | >111 | |
Concentration inhibiting enzymatic function by 50%.
Mean value ± standard deviation from triplicate experiments.
Ref 30.
