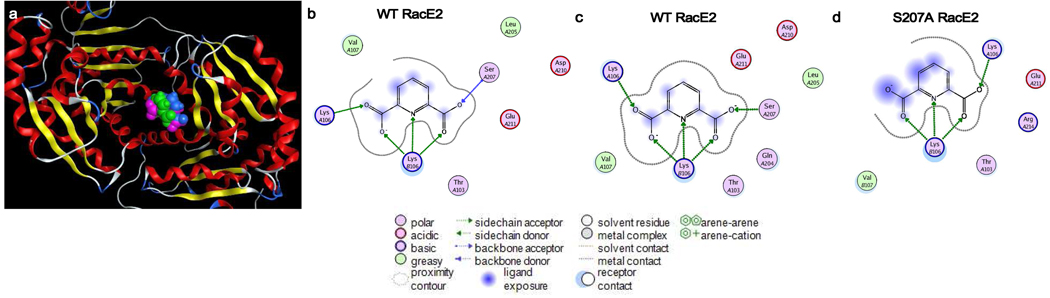
Figure 2.
Superpose of top-docked positions of DPA (space-filling) to the RacE2 dimer (ribbon, 2GZM) as determined by GOLD v4.1 (magenta), Autodock v4 (blue) and FRED v2.2.5 (green) (a). The binding pocket is located at the dimer interface and is composed of residues from both monomers, as detailed by the interaction map (b). After minimization, the backbone contact of Ser207 is swapped for a contact with the beta-hydroxyl group (c). After MD simulation of the top docked complex with Ser207 replaced by Ala, the binding site lacks any contact with the region previously containing Ser207 (d). Letters immediately preceding the residue numbers indicate the monomer, A or B. Ligand interaction maps were constructed using the LigX function of MOE v2009.10.