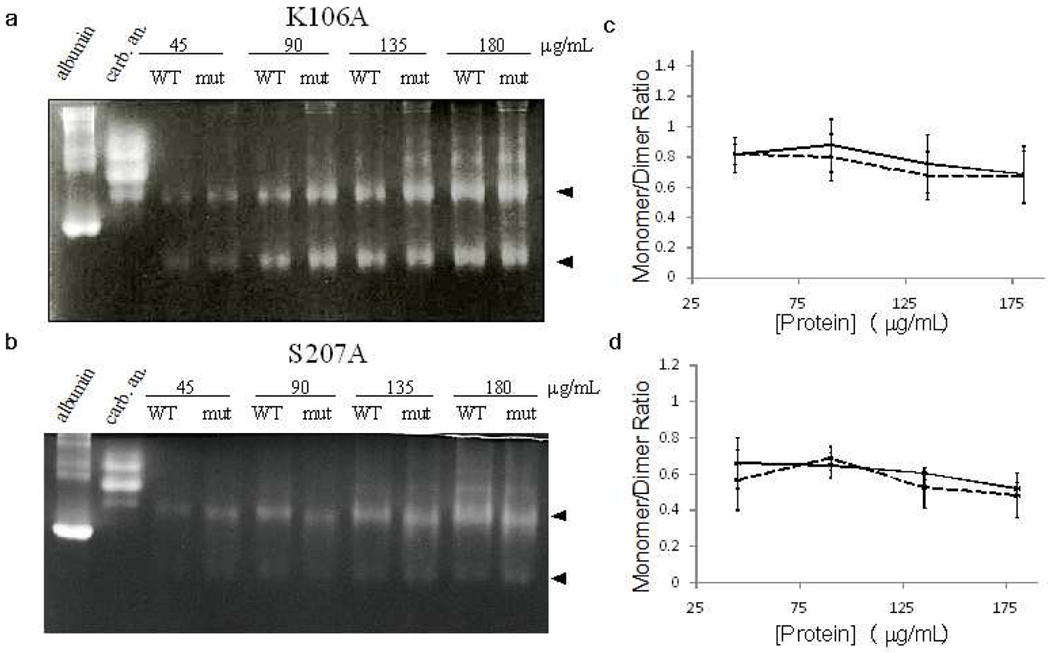
Figure 4.
BN-PAGE to determine the oligomerization of wild-type and mutant RacE2. Wildtype RacE2 and RacE2_S207A (a) or RacE2_K106A (b) were run side-by-side at concentrations varying from 45 to 180 µg/mL. Albumin and carbonic anhydrase were included as running controls. Arrowheads indicate bands representing the dimer and monomer. Band intensity was quantified via pixel counting and the ratio of monomer to dimer was plotted against protein concentration for RacE2_S207A (solid line = WT, dotted line = mutant; c) and RacE2_K106A (d). Data represents an average of three or more independent trials with standard error shown. Data was additionally fitted to the expression for M/C ratio as a function of total protein concentration and the monomer:dimer equilibrium constant (see Supplementary Methods for derivation of this expression and model fitting parameters).The results indicate that the two mutants do not have any significant effect on the oligomeric equilibrium. Additionally, see Figure S3 for BN-PAGE of RacE2 and running controls with NativeMark ladder.