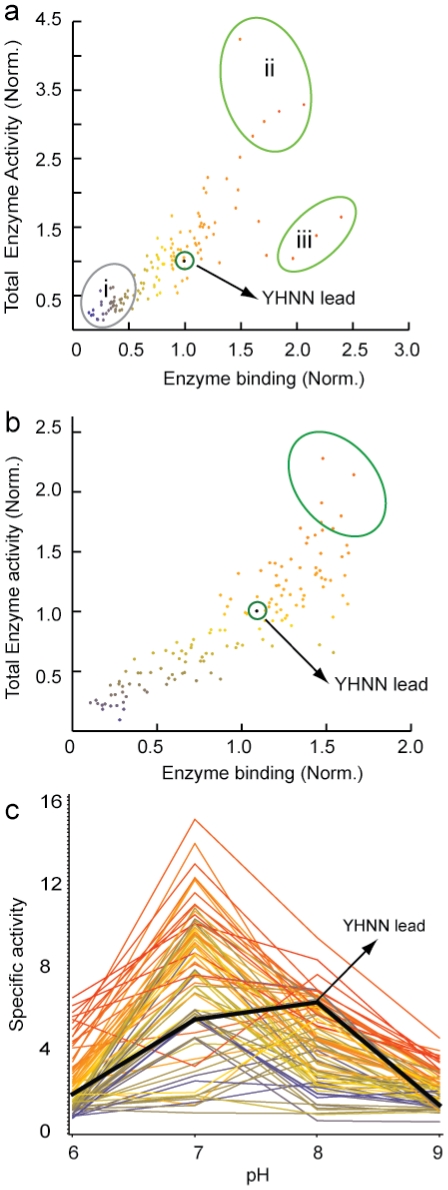
Figure 2. Point-variant screening of a lead peptide, YHNN.
β-Gal was bound to a microarray containing 132 YHNN variants and its activity was measured. (a) The activity of bound β-Gal on microarrays as a function of the amount of enzyme bound to a particular variant feature at room temperature. (i) Variants with poor affinity and activity; (ii) Variants with stronger affinity and higher activity; (iii) Variants with stronger affinity but relative lower activity. All data is normalized to the binding and activity values for the lead peptide, YHNN. (b) Thermal-stability assay. β-Gal was bound to the microarray containing YHNN variants as in (a) at room temperature, followed by incubation in phosphate buffer at 55°C for one hour. Enzyme activity was then assayed at room temperature. The selection region (circled) contains variants that bind to the enzyme with higher relative specific activity (the ratio of binding to activity) under thermal stress compared to YHNN after incubation at high temperature. (c) pH activity range assay. YHNN variant microarrays were bound to β-Gal as in (a) and incubated at room temperature in buffers with pHs ranging from 6 to 9 for one hour and then assayed for activity at the pH of incubation. The black line is the specific activity of β-Gal bound to the lead peptide, YHNN.