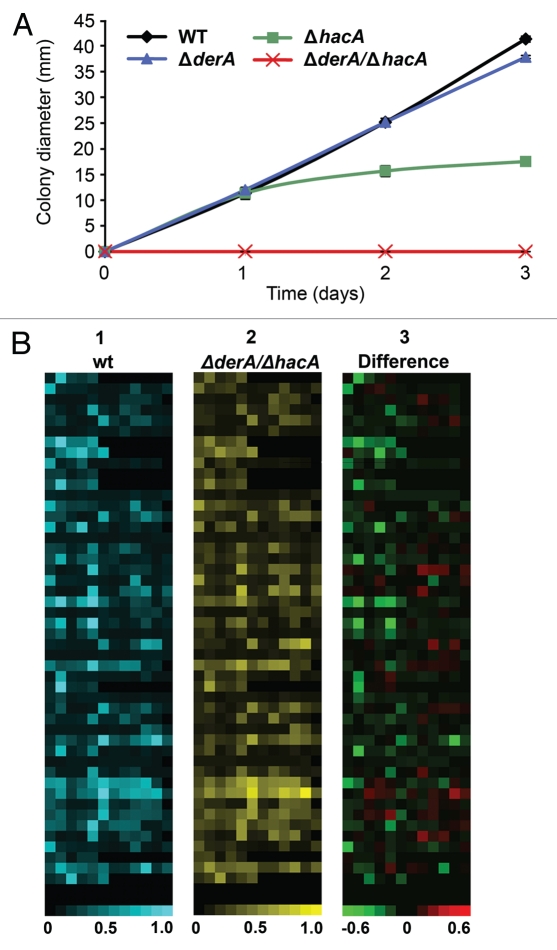
Figure 4.
DerA cooperates with the UPR to support protease secretion and growth on a complex substrate. (A) Growth on skim milk agar. Conidia from the indicated strains were inoculated onto the center of a plate of skim milk agar and colony diameter was monitored for 3 d at 37°C. (B) Analysis of secreted protease activity using a library of fluorescence resonance energy transfer (FRET) labeled peptide substrates. Equimolar mixtures of up to 8 individual FRET peptides in each well of a microtiter plate were incubated with culture supernatants from the indicated strains and heat maps were generated from the fluorescent signals generated by substrate cleavage, with each square corresponding to a single assay well. For part 1 (wt; blue) and part 2 (ΔderA/ΔhacA; yellow), the brightness of each square corresponds to the fluorescence intensity, quantified as fluorescence fold change, which indicates the extent of cleavage of the FRET peptides in that well. For part 3, the brightness of a given well indicates the extent to which specific protease activities are disproportionally decreased (green) or increased (red) in the ΔderA/ΔhacA mutant as compared to wt.