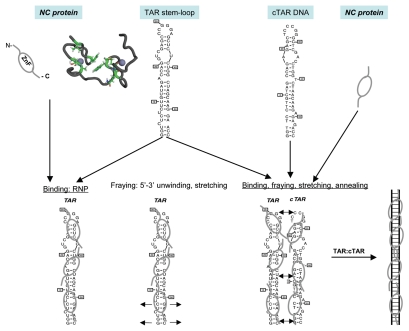
Figure 1.
Schematic illustration of the nucleic acids binding, fraying and annealing properties of the HIV nucleocapsid protein. Top, from left to right: NC protein is represented by an oval corresponding to the hydrophobic plateau (ZnF), flanked by the disordered N- and C-terminal domains shown here as small fragments. The 3D structure of the hydrophobic plateau at the top of the NC zinc-fingers is represented in the CPK mode with Val13, F16, Ile24, Ala25, Trp37, Gln45 and Met46, and the zinc atoms appear as grey spheres. The TAR with its loop and bulges is shown here as a stem-loop. The complementary DNA sequence is synthesized by RT chaperoned by NC at the very beginning of reverse transcription (reviewed in ref. 13 and 14) and shown here as cTAR stem-loop. Bottom, from left to right: Binding of NC to TAR results in the formation of a ribonucleoparticle where the RNA is entirely coated by NC molecules. This causes the transient opening of the 5′ and 3′ end sequences (short arrows), in a process called fraying and strand stretching (Single DNA molecule stretching measures the activity of chemicals that target the HIV-1 nucleocapsid protein).53 Binding of NC to TAR and cTAR causes the formation of nucleoprotein complexes where the nucleic acids are entirely coated by NC molecules. This causes the rapid hybridization of the complementary sequences resulting in the formation of a RNA:DNA hybrid within the nucleoprotein complex. This annealing reaction recapitulates the obligatory 5′-3′ strand transfer that occur at the beginning of reverse transcription.4