Figure 2.
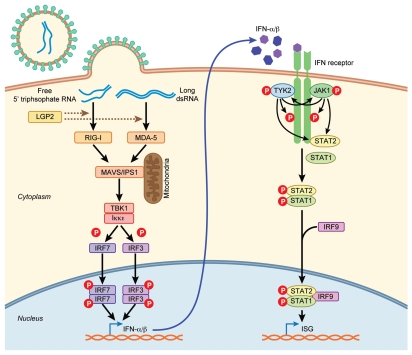
RNA helicases of the RIG-I-like receptor (RLR) family play a crucial role in the innate antiviral response. RLR family members, retinoic acid-inducible gene 1 (RIG-I/DDX58) and melanoma differentiation-associated gene 5 (MDA-5) are cytoplasmic sensors that detect viral RNA and trigger induction of the antiviral state. RIG-I and MDA-1 recognize viral RNA as foreign by features including free 5′ triphosphate ends, short double-stranded RNA (dsRNA) and long dsRNA (≥1 kb), respectively. RLR family member, laboratory of genetics and physiology 2 (LGP2) can antagonize this activity (dashed line) by regulation that is poorly understood. Interaction with their RNA ligands induces conformational changes in RIG-I and MDA-5 that favor interaction with mitochondria antiviral signaling (MAVS), also called interferon-beta promoter stimulator 1 (IPS-1). MAVS/IPS-1 activates the TANK-binding kinase 1 (TBK1)/IκB kinase-ε (IKKε) complex, which phosphorylate IFN-regulatory factor (IRF)-3, IRF-7 and NFκB (not shown). The IRFs dimerize, translocate to the nucleus and bind to target genes. Transcriptional activation of interferon (IFN) genes leads to production of IFNs (α and β), which are secreted from the cell. IFN binding to the IFN receptor activates the janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway. The JAK and tyrosine kinase 2 (TYK2) phosphorylate one another, the receptor and STAT2. STAT2 binds STAT1 and triggers phosphorylation and STAT1/2 dimerization. The STAT heterodimer translocates to the nucleus with IRF-9. They activate transcription of interferon stimulated genes (ISG), which generate the anti-viral state that attenuates viral replication.