Summary
IFNα is known to play a critical role in the pathogenesis of systemic lupus erythematosus (SLE), but the mechanisms remain unclear. We previously showed that within weeks, exposure to IFNα in vivo induces lupus in pre-autoimmune lupus-prone NZB × NZW F1 (NZB/W) but not in BALB/c mice. In the current study, we show that in vivo expression of IFNα induces sustained B cell proliferation in both BALB/c and NZB/W mice. In NZB/W but not BALB/c mice, B cell proliferation was accompanied by a rapid and unabated production of autoantibody-secreting cells (ASCs) in secondary lymphoid organs, suggesting that a B cell checkpoint is altered in the autoimmune background. The majority (>95%) of ASCs elicited in IFNα-treated NZB/W mice were short-lived and occurred without the induction of long-lived plasma cells. A short course of cyclophosphamide caused a sharp drop in IFNα-elicited short-lived plasma cells, but the levels recovered within days following termination of treatment. Thus, our work provides new insights into effectiveness and limitations of current SLE therapies.
Keywords: lupus, interferon alpha, B lymphocytes, short-lived plasma cells, cyclophosphamide
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by inflammation in multiple organs and the presence of anti-nuclear autoantibodies, some of which, especially anti-dsDNA, are associated with tissue pathogenicity [1]. The type I IFN system is thought to play a pivotal role in human SLE [2]. In addition, studies in mouse models of SLE have revealed a major role for this cytokine in the pathogenesis of the disease. Indeed, NZB and C57/Bl6 lpr/lpr mice lacking IFNAR-1, the α-chain of the common receptor for type-I IFNs, have reduced lupus-like disease [3-4]. Furthermore, we recently showed that prolonged expression of IFNα in vivo rapidly induces a dramatic and full-blown lupus in pre-autoimmune NZB × NZWF1 (NZB/W) mice but not in BALB/c mice [5], supporting the idea that type I IFNs play a pivotal role in disease severity and onset [6].
Major alterations in the B cell compartment, including selection, altered localization, phenotypes, and hyperactivation, as well as defects in apoptosis and in checkpoints regulating splenic plasma cell accumulation and Ig production, are thought to contribute to the production of pathogenic autoantibodies responsible for end-organ damage in SLE [7-9]. Type I IFNs have a variety of effects on the function and development of B cells that are reminiscent of B cell alterations in SLE [10]. For example, type I IFNs increase B cell proliferation and their resistance to apoptosis [11-13] and can increase Ig secretion by B cells [14]. Furthermore, activated B cells exposed to IFNα and IL-6 released by virus-activated plasmacytoid dendritic cells differentiate into highly efficient Ig-secreting cells [15]. In addition, in vivo, IFNα and β are potent enhancers of antibody responses and Ig isotype switching [16-17]. Accordingly, lupus-prone mice deficient in the type I IFN receptor have reduced levels of circulating Ig [4].
IFNα and β may also be responsible for the increased frequency of antibody-secreting cells (ASCs) in SLE [18-19]. Short-lived plasmablasts and long-lived plasma cells, which are commonly refractory to immunosuppressive treatments coexist in the lupus-prone model, NZB/W, and both seem to contribute to chronic humoral autoimmunity [20-21]. Plasmablasts occur at high frequencies in the circulation of active but not inactive SLE patients [18-19, 22-23], but their contribution to the autoimmune process remains unclear. Various experimental studies suggest that long-lived autoimmune plasma cells also exist in humans [24], and whether IFNα and β drive B cell differentiation into short-lived or long-lived ASCs in lupus may have important therapeutic implications.
Our previous studies showed that sustained adenovirus-mediated expression of IFNα in vivo induces early lethal lupus with severe immune complex glomerulonephritis in pre-autoimmune lupus-prone, NZB/W mice but not in BALB/c mice [5]. Here, we used these two mouse strains to investigate the effects of long-term expression of IFNα in vivo on B cell activation and production of ASCs in autoimmune and normal backgrounds.
Results
Expression of IFNα in vivo induces the sustained production of ASCs in young NZB/W but not in BALB/c mice
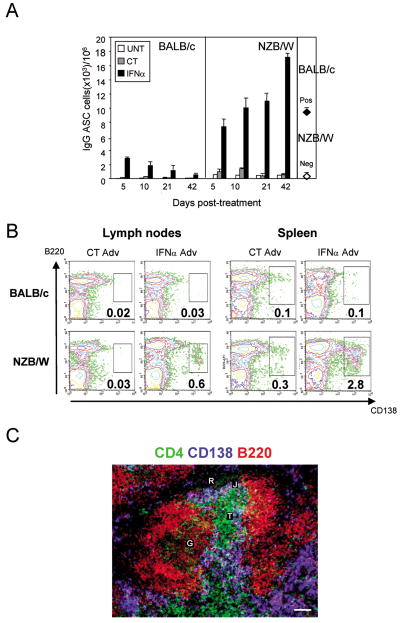
To examine whether IFNα enhances the production of ASCs in preautoimmune, lupus-prone mice, NZB/W and BALB/c controls were injected with a recombinant adenovirus vector containing the murine IFNα subtype 5 cDNA (IFNα Adv) or a control virus (CT Adv). ELISPOT experiments revealed that in the absence of IFNα Adv treatment, levels of ASCs were higher in the secondary lymphoid organs of NZB/W mice compared to BALB/c mice (10- to 20-fold higher in spleen and 5-fold higher in lymph nodes). Within a few days after treatment initiation, IFNα Adv enhanced the frequency of total IgG ASCs in the spleen (A) and lymph nodes (data not shown) of both BALB/c and NZB/W mice to a similar extent (11- to 16-fold in the spleen and 65- to 132-fold in lymph nodes). The initial increase in IgG ASCs was followed by a gradual decline in BALB/c mice, whereas there was continued increase in NZB/W mice (up to 30-fold in the spleen and 80-fold in lymph nodes vs. control on day 42; data not shown). IFNα expression did not significantly increase IgG ASC levels in the bone marrow (data not shown).
Expression of CD138, an ASC marker [25], confirmed that IFNα caused a dramatic increase in the frequency (B) and absolute numbers (Supplemental Figure 2) of CD138+B220low cells in both the spleen and lymph nodes of NZB/W compared to BALB/c mice. We confirmed that more than 95% of CD138+B220low cells were positive for intracellular Ig, which was not detected in CD138- cells (data not shown). Immunofluorescence on spleen sections from IFNα-treated NZB/W mice showed that there were many CD138+ cells scattered throughout the red and the white pulp (C and Supplemental Figure 3). These CD138+ cells were localized in the periarteriolar lymphoid sheath and at the junction of the T-cell zone and the red pulp (C), consistent with early ASCs [26]. Furthermore, as in old NZB/W mice, which have full-blown lupus, these IFNα-elicited cells were characterized by the expression of IgG2a and IgG3 (Supplemental Figure 4). These cells displayed autoreactivity for ssDNA, dsDNA, nucleosomes and total histones, and low but significant autoreactivity towards α-actin and phosphatidyl serine (Supplemental Figure 4). Finally, in both strains, IFNα treatment in vivo increased the circulating levels of IL-6 and TNFα, two cytokines involved in ASC differentiation and survival [27] (Supplemental Figure 5).
IFNα expression in vivo sustains the proliferation of GC B lymphocytes and plasmablasts in NZB/W but not BALB/c mice
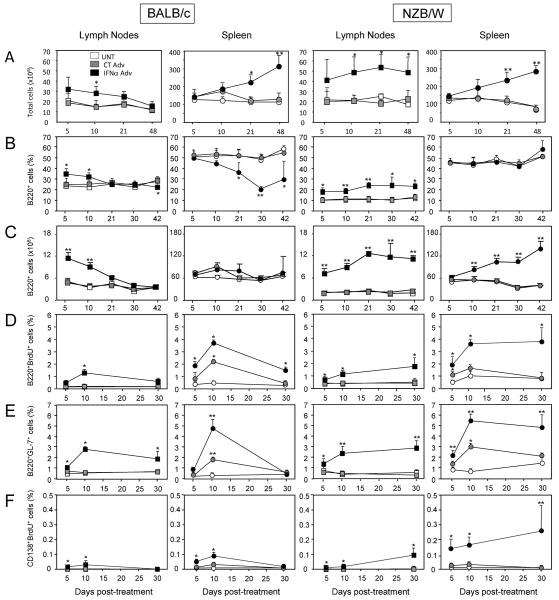
IFNα treatment in vivo elicited an increase in leukocyte cellularity in the spleen of both Balb/c and NZB/W mice (approximately 2- to 3-fold vs. CT Adv mice) (Fig. 2A). There was also a prominent increase in leukocyte cells in LNs of IFNα Adv-treated NZB/W mice (Fig. 2A), whereas the numbers increased only transiently, early after initiation of treatment, in Balb/c mice. We further investigated how IFNα affects the different B cell compartments. Treatment of BALB/c mice with IFNα Adv caused an early increase in total B cell frequency in lymph nodes that returned back to control levels within 3 weeks post-treatment (B, left). In the spleen, B cell frequency declined with time, reaching 21% of total cells (vs. 50% in CT Adv mice; p=0.002) at 30 days post-treatment. This B cell lymphopenia was relative and not absolute (Fig. 2C) and resulted from a decrease in the frequency of all splenic B cell subsets (i.e., transitional T1 and T2, marginal zone, and follicular; data not shown). Treatment of NZB/W mice with IFNα Adv rapidly increased lymph node B cell frequency up to day 21, reaching 23.7±0.9% of total cells (vs. 11.0±2.2% in CT Adv mice; p<0.005). Thereafter, the high prevalence of B cells remained stable up to 42 days post-treatment (B, right). IFNα treatment did not induce B cell lymphopenia (B, right) or change s in B cell subset frequencies in the spleens of these mice (data not shown).
Figure 2. IFNα elicits sustained B lymphocyte and plasmablast proliferation as well as GC formation in young NZB/W mice but not in Balb/c mice.
(A) White cell counts in secondary lymphoid organ in Balb/c and NZB/W mice. (B) Frequency and (C) absolute number of B220+CD19+ B cells in the spleen and lymph nodes of Balb/c and NZB/W mice as determined by FACS. (D) Proliferation of B cells was assessed by FACS of B220+BrdU+ cells from mice injected with BrdU 2-3 h before sacrifice at the indicated times. (E) Frequency of GC B cells was determined by FACS of B220+GL-7+ cells. (F) Frequency of proliferating plasmablasts was determined by FACS of B220lowCD138+BrdU+ cells from mice injected with BrdU 2 to 3 h before sacrifice at the indicated times. Results are means ± SD of 3 to 5 mice/experimental group from 2 independent experiments and are expressed as % of total cells analyzed. Comparisons between IFNα Adv-treated and CT Adv-treated animals and between CT Adv-treated and untreated animals were made using the Mann-Whitney U test. *, p<0.05; **, p<0.01.
Within 10 days after initiation of IFNα treatment, the prevalence of GC B cells (B220+GL-7+) increased 2- to 3-fold (vs. CT Adv mice) in the spleen and lymph nodes of both BALB/c and NZB/W mice (E). At late time points (i.e., day 30), however, IFNα-induced GC B cell activation fell in BALB/c mice back to levels found in control mice, while, in contrast, the elevated GC B cell frequency remained stable in IFNα treated-NZB/W mice.
Analysis of B cell proliferation using a short (2-h) pulse of BrdU revealed that IFNα expression enhanced B cell proliferation in the spleen and lymph nodes of both BALB/c and NZB/W mice (D) at day 5 after initiation of treatment. At day 30, B cell proliferation stabilized in the spleen and lymph nodes of NZB/W mice (>4-fold increase in the spleen vs. CT Adv; p<0.005), whereas it decreased in the lymph nodes and spleen of BALB/c mice. Interestingly, within days after initiation of IFNα treatment, B cells from both mouse strains also proliferated as plasmablasts (BrdU+CD138+; i.e., dividing CD138+ cells; F). The frequency of plasmablasts continued to increase up to day 30 in NZB/W mice while decreasing in BALB/c to levels observed in control mice. Notably, at all time-points, plasmablasts represented 14% to 18% of total CD138+ cells in both the spleen and lymph nodes of IFNα Adv-treated NZB/W mice (data not shown).
IFNα drives unabated production of ASCs in young NZB/W mice
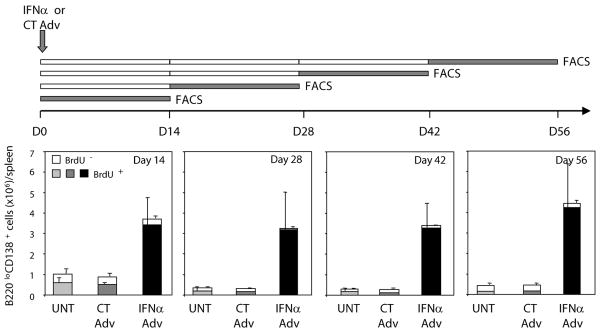
We next examined whether the sustained B cell proliferation induced by IFNα in NZB/W mice is associated with continuous production of new ASCs (A). Four groups of mice treated at day 0 with IFNα Adv or CT Adv were fed BrdU for 14 consecutive days but beginning at different time points (i.e., day 0–14, 14–28, 28–42, and 42–56; Figure 3, top) so as to cover the entire duration of IFNα-mediated disease. At the end of each BrdU feeding period, spleens were harvested and BrdU incorporation was assessed by FACS in gated CD138+B220low cells. In each group of mice, the vast majority (>90%) of ASCs detected at the end of the BrdU feeding period were BrdU-positive (Figure 3, bottom), indicating that the ASCs were generated during the 14-day labeling period. Similar results were found using cells isolated from lymph nodes (data not shown). Thus, long-term expression of IFNα in vivo induces unabated production of ASCs in NZB/W mice.
Figure 3. IFNα in vivo promotes the continuous generation of ASCs.
Four groups each of untreated (UNT), CT Adv-treated (CT), or IFNα Adv-treated (IFNα) NZB/W mice (at day 0) were fed BrdU for different 14-day periods (days 0-14, 14-28, 28-42, and 56-70) at the end of which mice were sacrificed and spleen cells were stained for intracellular BrdU (top panel), and BrdU staining of gated B220loCD138+ cells were analyzed by FACS. Raw results (see Supplemental Figure 6) were converted into absolute numbers of BrdU-negative (white columns) and BrdU-positive (shaded columns) B220loCD138+ cells/spleen and are the means ± SD of 3 to 4 mice per group based on three independent experiments.
IFNα-elicited ASCs are short-lived cells
The bone marrow, a likely destination for ASC emigrants [28], did not show significant increases in ASCs upon treatment with IFNα (data not shown), and almost no IgG ASCs were present in the kidney, liver, peritoneal cavity and the gut from IFNα-treated NZB/W mice (data not shown). On the contrary, TUNEL counterstaining of CD138+ cells to assess apoptosis indicated that, at day 48 after initiation of IFNα treatment, 7.2 ± 3.7% of splenic CD138+ cells were apoptotic (vs. 2.6 ± 2.0% in CT Adv-treated mice) (Supplemental Figure 6). This suggested that the IFNα-elicited ASCs are mainly short-lived cells.
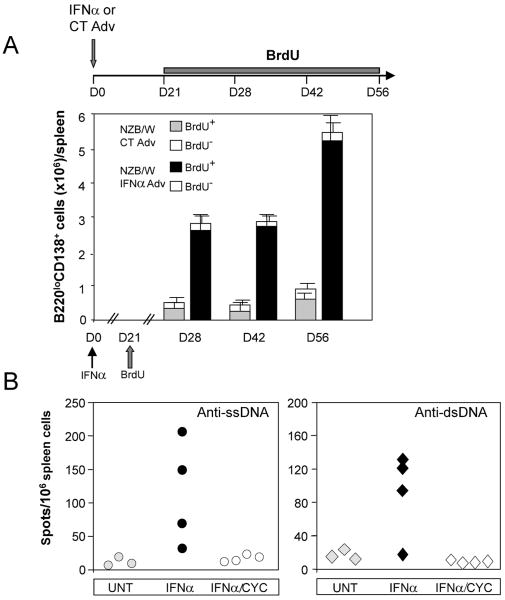
To further investigate the nature of the IFNα-elicited ASCs, we performed BrdU labeling experiments. Because approximately half of IFNα Adv-treated NZB/W mice die by day 60 [5], mice were continuously fed BrdU from day 21 and up to week day 56 (A, top), which is sufficient for detection of long-lived plasma cells [29]. The number of spleen BrdU-negative CD138+B220low cells was identical in mice treated with IFNα Adv and CT Adv. This indicates that IFNα expression in vivo does not enhance the production of long-lived plasma cells in the spleens of NZB/W mice (A, bottom).
To confirm that the anti-dsDNA ASCs elicited by IFNα in NZB/W mice are short-lived cells, we examined the sensitivity of the IFNα-elicited ASCs to a short course of cyclophosphamide [30], which should suppress the growth of short-lived cells but not affect pre-existing long-lived cells. Three weeks after initiation of IFNα treatment, NZB/W mice were injected with 20 mg/kg cyclophosphamide or PBS for 3 consecutive days and then sacrificed 4 days following the last injection. This treatment completely abolished IFNα-induced production of anti-ssDNA and anti-dsDNA ASCs in the spleen (B), confirming that they are short-lived cells.
Cyclophosphamide causes a transient remission of IFNα-induced ASC production
Finally, we examined whether cyclophosphamide can provide long-term inhibition of ASC production in IFNα-treated NZB/W mice. IFNα Adv-expressing mice were injected every day for 3 days with cyclophosphamide and then sacrificed four days after the last cyclophosphamide injection. This treatment caused the levels of B220loCD138+ ASCs to decrease to those detected in mice expressing CT Adv (left). However, the pool of IFNα-elicited GC B cells and ASCs partially recovered by day 11 after the last injection of cyclophosphamide and recovered entirely by day 25 (right). Thus, a short course of cyclophosphamide does not cause long-term suppression of IFNα-induced ASC production.
Discussion
We previously showed that prolonged expression of IFNα in vivo induces a rapid lethal lupus with immune complex glomerulonephritis in NZB/W lupus-prone mice, but not in normal BALB/c mice [5]. We now demonstrate that in NZB/W but not BALB/c mice, treatment with IFNα causes excessive and unabated production of short-lived autoreactive ASCs throughout the course of the disease.
IFNα treatment had markedly different effects on the B cell compartment of BALB/c and NZB/W. According to the kinetics of B cell proliferation and ASC production in vivo, the action of IFNα on the B cell compartment can roughly be separated into two distinct phases. During the early phase (i.e., days 5 to 10 post-treatment), B cell proliferation and ASC production occur at comparable levels in BALB/c and NZB/W mice, which likely reflects the enhancing effect of IFNα on Adv-mediated B cell proliferation and differentiation as seen in IFNα-treated mice immunized with a soluble antigen [16-17]. During the late phase of IFNα action in vivo (i.e., day 21 up to day 42 post-treatment), where B cell proliferation in CT Adv-treated mice falls to baseline levels in both mouse strains, B cell proliferation and differentiation are arrested in BALB/c cells. Conversely, NZB/W mice show strong B cell proliferation and dramatic ASC production until late in the disease course. Furthermore, IFNα elicits the production of ASCs with anti-nuclear reactivity exclusively in the lupus-prone strain. IFNα also induced relative B cell lymphopenia in the spleens of BALB/c mice. This effect was not observed in NZB/W mice and might be due to sustained B cell proliferation induced by IFNα in NZB/W mice (Figure 2D) as well as a higher resistance to apoptosis of NZB/W B cells compared to BALB/c B cells [31]. However, we were unable to detect such differences in vivo, and in both strains, B cell lymphopoïesis was decreased to the same level and for the same duration (our unpublished observations). The ability of IFNα to induce exaggerated and sustained B cell proliferation and ASC differentiation in NZB/W but not BALB/c mice might be related to the increased expression of Ifi202, an interferon-inducible transcription factor that controls cell-signaling pathways, regulates cell proliferation, survival, and differentiation, and leads to lupus by inhibiting lymphocyte apoptosis [32].
The ASCs elicited by IFNα in lupus-prone mice were found to be almost exclusively short-lived cells. Although the characteristics distinguishing short-lived plasmablasts from short-lived plasma cells are poorly defined [33], we feel, as others [34], that proliferation definitely separates dividing plasmablasts from non-dividing plasma cells. Our short-BrdU pulse experiments indicate that if a large fraction (14-18%) of ASCs was proliferating, reflecting a high turnover rate, an even larger fraction (>80%) was not. This latter pool of short-lived plasma cells might represent, at least in part, recent nondividing plasmablast siblings. The origin of these ASCs is not clear. In particular, whether they derive from extrafollicular plasmablast growth, GC reaction, or both remains to be elucidated. We indeed found GC B cell proliferation in the spleen of NZB/W mice treated with IFNα but did not find evidence for long-lived plasma cells, which are widely thought to derive from GC-activated B cells [35]. It has long been assumed that autoimmune ASCs that secrete high-affinity somatically mutated antibodies are derived from the GC reaction [36-38]. Recently, however, this concept has been challenged by the description of somatically mutated autoimmune rheumatoid factor B cells emerging outside the GC [39]. It is also known that plasmablasts exiting GCs do undergo cell division just before they become plasma cells [40-42] and IFNα might also act at a post-GC levels.
Several mechanisms could explain why ASCs, produced during the whole autoimmunity process are short-lived. IFNα might dramatically alter the microenvironment, constituted in part by adhesion molecules [43], IL-6 [44], CXCL12 [45], TNFα [27], BAFF [46], and APRIL [46], which normally favors survival niches for long-lived plasma cells (reviewed in [47]). To address this, we examined the induction of serum cytokines by IFNα, focusing on cytokines known to play a role in ASC differentiation and survival. We previously showed that IFNα induces BAFF in both the NZB/W and the BALB/c mice to the same extent [5]. We show here that IFNα also induces IL-6 and TNFα in both strains and that BAFF, IL-6, and TNFα participate also in the enhancement of B cell proliferation and ASC differentiation seen in IFNα-treated mice. Furthermore, we found that treatment with IFNα results in less IL-6 and more TNFα secretion in NZB/W mice than in BALB/c mice. This loss of balance between IL-6 and TNFα probably alters the microenvironment surrounding ASCs in a way so that the long-term survival of ASCs is no longer supported. It is also possible that due to the spleen's finite capacity to sustain plasma cell survival [41], a dramatic influx of newly IFNα-elicited plasma cells might overwhelm the niches in the spleen where plasma cells normally accumulate and survive for long periods [20-21].
Defining the nature of the ASCs and therefore the origin of autoantibodies is critical for the interpretation of existing and emerging clinical data as well as for the future development of targeted treatments. Indeed, it is thought that long-lived plasma cells but not short-lived plasmablasts are resistant to immunosuppressive treatments and may be responsible for the persistent autoantibody levels in treated patients (reviewed in [24, 47]). Different ASCs populations, namely, long- and short-lived ASCs, may participate in the pathogenesis of lupus. In SLE patients plasmablasts are found in high numbers in the circulation of SLE patients [18-19], and their titers correlate with disease activity [22-23]. Nonetheless, various studies suggest that long-lived plasma cells also exist in humans and contribute to the production of autoantibodies [24]. Long-lived plasma cells have been found to co-exist with short-lived plasma cells/plasmablasts in the spleen of spontaneously diseased NZB/W mice [21]. Treatment of these mice with cytotoxic drugs such as cyclophosphamide caused a marked reduction in ASCs [48], but these drugs are unable to target the compartment of autoantibody-secreting long-lived plasma cells [21]. Moreover, like SLE patients, mouse models of lupus display heterogeneous disease phenotypes. Indeed, transgenic lupus-prone mice in the MRL background show plasmablasts to dominate the early and late spontaneous rheumatoid factor response [34, 39]. Thus, new therapeutic treatments await further detailed characterization of the pathogenic effector cells involved in the autoimmune reaction. Nonetheless, our data show that cyclophosphamide acts in part by blocking B cell proliferation and ensuing ASCs production. The pool of IFNα-driven ASCs, although abolished by a short-pulse of cyclophosphamide, was rapidly reconstituted after discontinuation of this drug (e.g., 50% after 11 days). Therefore, it is possible that the persistence of autoantibodies in SLE patients under immunosuppressive therapy could be due to the inability of cyclophosphamide to shut off a chronically IFNα-driven activated B cell response and the ensuing production of autoreactive short-lived plasma cells. Extrapolating these data to treatment approaches in humans must be done with caution, but they indicate that it may be possible to design specific therapies aimed at neutralizing the chronic stimulatory effects of IFNα on ASC generation.
In conclusion, our study helps clarifying the role of IFNα in the pathogenesis of lupus. We show that autoimmunity and development of overt disease can be driven by short-lived ASCs. Analysis of the origin and nature of ASCs in control and relevant SLE models may provide tools for understanding the effectiveness and limitations of currently available SLE therapies.
Materials and Methods
IFNα adenoviruses
IFNα Adv was obtained from QBiogene (Carlsbad, CA), and Control Adv (CT Adv) was generated from IFNα Adv as described previously [5].
Mice and in vivo expression of IFNα
NZB/W mice were raised at the Baylor Institute for Immunology Research in a specific pathogen-free barrier facility from breeders purchased from Harlan (Indianapolis, IN). Age-matched BALB/c mice (Harlan) were used as non-autoimmune controls. Ten week-old female mice received a single retro-orbital intravenous injection of 1010 adenovirus particles as described previously [5]. All in vivo experiments were approved by the Baylor Research Institute Institutional Animal Care and Use Committee.
FACS
Single-cell suspensions of spleen and lymph nodes were obtained by standard procedures. All blocking and staining procedures were performed with mAbs from BD Pharmingen (San Diego, CA) unless otherwise specified. Fc receptors were blocked with anti-CD16/CD32 (40 μg/ml), after which B lymphocytes were detected using FITC-anti-CD19 (clone 6D5) (Caltag, Burlingame, CA) and APC-anti-B220 (clone RA3-6B2), GC B cells with FITC-anti-GL-7, and plasmablasts/plasma cells with PE-anti-syndecan/CD138 (clone 281.2). After blocking of surface Ig and Fc receptors with a mixture of unlabeled anti-mouse IgM/IgG isotype and anti-CD16/CD32 and permeabilization of cell membranes with Cytofix/Cytoperm solution (BD Pharmingen), intracellular IgM/IgG was detected using FITC-conjugated anti-mouse IgM or isotype-specific IgG mAbs. FACS was performed using a FACScalibur cytometer and Cell Quest software (BD Pharmingen).
ELISPOT assays for the quantitation of antibody-producing B cells
Single-cell preparations from spleen, lymph node, bone marrow, liver, gut, kidney, and peritoneal cavity were obtained by standard procedures except that red blood cell lysis was omitted. Ninety-six-well plates with polyvinylidene diflouride filters (Millipore Multiscreen; Bedford, MA) were coated overnight at 4°C with anti-mouse IgG or IgM (3 μg/ml; Jackson ImmunoResearch, West Grove, PA). The plates were washed with PBS and blocked with PBS/3% BSA for 2 h at room temperature. Three-fold serial dilutions of cells in growth medium (RPMI supplemented with 5% fetal calf serum, antibiotics and 10-5 M β-mercaptoethanol) were plated starting at 106/well. After 5 h at 37°C, plates were washed with PBS/3% BSA/0.01% Tween-20. Next, biotin-conjugated anti-mouse IgM (1 μg/ml in PBS) or a mixture of biotin-conjugated anti-mouse IgG isotypes (IgG1, IgG2a, IgG2b, and IgG3; 1 μg/ml each) were added. After 40 min at room temperature, plates were washed and streptavidin-HRP (1:1000; BD Pharmingen). After 20 min, plates were developed with Opti4CN (Bio-Rad; Hercules, CA). Spots were counted using an ELISPOT reader equipped with Immunospot 5.1 software (CTL Immunospot; Cleveland, OH). Anti-ssDNA/dsDNA antibody-secreting plasma cells were counted using the same protocol except that multiscreen plates were first coated with ssDNA (5 μg/ml) or methylated BSA (1 μg/ml; Sigma) followed by dsDNA (5 μg/ml). To obtain ssDNA, dsDNA (Roche Diagnostics, Indianapolis, IN) was boiled and rapidly chilled.
Immunofluorescence microscopy
Triple-staining for plasma cells, CD4+ T cells, and B cells was performed using unconjugated anti-CD138 followed by Cy5-conjugated anti-rat Abs and then FITC-conjugated anti-CD4 and biotinylated anti-B220 followed by streptavidin-Alexa 568. Fluorescent images were captured using a BX-61 upright fluorescent microscope (Olympus) equipped with a Cool Snap HQ CDD camera (Photometrics). Post-acquisition imaging was processed using Metamorph software version 6.1 (Universal Imaging, Downington, PA).
Analysis of BrdU incorporation
Mice were injected i.p. with 2 mg of BrdU (Sigma Aldrich) 2 h before sacrifice or were given BrdU in drinking water (1 mg/ml in 1% glucose) that was protected from light and changed every day. Splenocytes were treated to block Fc receptors and stained with PE-anti-CD138, APC-anti-B220, and BrdU using a BrdU-Flow-Kit (BD Pharmingen). BrdU staining was analyzed by FACS on gated B220loCD138+ cells.
Cyclophosphamide treatment
Starting 21 days after initiation of IFNα treatment, NZB/W mice were treated for three consecutive days with 20 mg/kg cyclophosphamide (Cytoxan®; MeadJohnson, Princeton, NJ) by i.p. injection.
Statistical analysis
Experimental results were analyzed for statistical significance using the Mann-Whitney U test. Differences were considered statistically significant when the p-value was below 0.05.
Supplementary Material
Figure 1. IFNα in vivo elicits long-term production of ASCs in NZB/W but not in BALB/c mice.
(A) Frequency of spleen IgG ASCs was determined by ELISPOT. Results are the means ± SD of three mice in each group from two independent experiments. Neg, young untreated NZB/W mice; pos, old proteinuric untreated NZB/W mice; UNT, untreated; CT, control adenovirus (CT Adv)-treated; IFNα, interferon alpha (IFNα Adv)-treated. (B) Spleen and lymph node cells were stained with fluorescent anti-B220 and anti-CD138 Abs, and the frequency of ASCs analyzed by FACS at day 42 post-treatment. Data shown are representative of eight experiments (C) Immunofluorescence staining of plasma cells in spleen sections from IFNα-treated NZB/W mice for CD4 (green), CD138 (blue), and B220 (red). T, T cell zone; J, junction of the T cell zone; R, red pulp; G, germinal center. Data shown are representative of four experiments.
Figure 4. IFNα treatment elicits short-lived ASCs in NZB/W mice.
(A) Absence of long-lived plasma cells. NZB/W mice were treated at day 0 with CT or IFNα Adv and then continuously fed BrdU for 35 days starting at day 21 post-treatment. Mice were sacrificed at indicated times, and BrdU staining of B220lowCD138+ cells was analyzed by FACS. Results are means ± SD of 3 to 5 mice per experimental group from two independent experiments (B) NZB/W mice were untreated (UNT) or treated with IFNα Adv and either with PBS (IFNα) or with 20 mg/kg cyclophosphamide (IFNα/CYC) on 3 consecutive days starting on day 21 after initiation of IFNα treatment. Mice were sacrificed 4 days after the last injection of cyclophosphamide, and spleen anti-ssDNA (left) or anti-dsDNA(right) ASCs were measured by ELISPOT. Each symbol represents an individual mouse. Data shown are representative of three independent experiments.
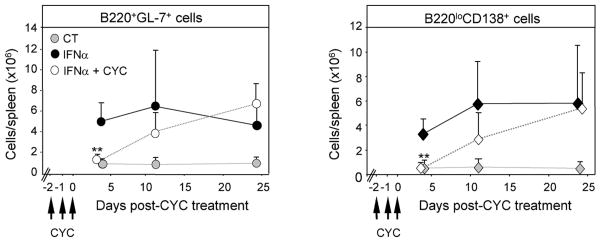
Figure 5. Effect of a short course of cyclophosphamide on IFNα-induced B cell proliferation and ASC production.
IFNα Adv-treated NZB/W mice were injected with 20 mg/kg cyclophosphamide (IFNα+CYC) for 3 consecutive days starting at 21 days after initiation of IFNα treatment. Controls consisted of CT Adv-treated NZB/W mice (CT) and IFNα-Adv-treated NZB/W mice that had not received cyclophosphamide (IFNα). At indicated times, the numbers of spleen B220+GL-7+ GC B cells and B220loCD138+ ASCs were determined by FACS. Results are the means ± SD of 3 to 5 mice per group based on three independent experiments. Comparisons between IFNα Adv-treated and IFNα Adv + CYC-treated animals were made using the Mann-Whitney U test. **, p<0.01.
Acknowledgments
The authors thank Professor F. Hiepe (Charité Universitätmedizin Berlin) for technical assistance with anti-dsDNA ELISPOT assays and Dr. Robert Gerard (University of Texas Southwestern) for advice on the use of adenoviruses. We also thank S. Clayton, E. Krauss, K. Joseph, and J. Zazoun for technical assistance; Drs. G. Jego, I. Gresser, D. Nochy, and D. Damotte for discussions; and Drs D. Emilie and A. Dalloul for support and critical reading of the manuscript; and Dr. P. Leventhal (4 Clinics, France) for assistance in preparing this manuscript for publication. The work described here was supported in parts by grants to J.B. from the Baylor Health Care System Foundation, the Alliance for Lupus Research, the Center for Lupus Research (AR054083), and the NIH (AI068842).
Abbreviations used
- ASC
antibody secreting cell
- CT Adv
adenovirus lacking a cDNA insert
- IFNα Adv
recombinant adenovirus vector containing the murine IFNα subtype 5 cDNA
- NZB/W
NZB × NZW F1
- SLE
systemic lupus erythematosus
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
References
- 1.Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 2.Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54:408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 3.Braun D, Geraldes P, Demengeot J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 4.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2:764–766. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 8.Hagn M, Ebel V, Sontheimer K, Schwesinger E, Lunov O, Beyer T, Fabricius D, Barth TF, Viardot A, Stilgenbauer S, Hepp J, Scharffetter-Kochanek K, Simmet T, Jahrsdorfer B. CD5+ B cells from individuals with systemic lupus erythematosus express granzyme B. Eur J Immunol. 40:2060–2069. doi: 10.1002/eji.200940113. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez T, Halcomb KE, Coughran AJ, Li QZ, Satterthwaite AB. Separate checkpoints regulate splenic plasma cell accumulation and IgG autoantibody production in Lyn-deficient mice. Eur J Immunol. 2010;40:1897–1905. doi: 10.1002/eji.200940043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 11.Morikawa K, Kubagawa H, Suzuki T, Cooper MD. Recombinant interferon-alpha, -beta, and -gamma enhance the proliferative response of human B cells. J Immunol. 1987;139:761–766. [PubMed] [Google Scholar]
- 12.Su L, David M. Inhibition of B cell receptor-mediated apoptosis by IFN. J Immunol. 1999;162:6317–6321. [PubMed] [Google Scholar]
- 13.Braun D, Caramalho I, Demengeot J. IFN-alpha/beta enhances BCR-dependent B cell responses. Int Immunol. 2002;14:411–419. doi: 10.1093/intimm/14.4.411. [DOI] [PubMed] [Google Scholar]
- 14.Neubauer RH, Goldstein L, Rabin H, Stebbing N. Stimulation of in vitro immunoglobulin production by interferon-alpha. J Immunol. 1985;134:299–304. [PubMed] [Google Scholar]
- 15.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 16.Finkelman FD, Svetic A, Gresser I, Snapper C, Holmes J, Trotta PP, Katona IM, Gause WC. Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J Exp Med. 1991;174:1179–1188. doi: 10.1084/jem.174.5.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 18.Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol. 2001;167:2361–2369. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- 19.Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, Lipsky PE, Radbruch A, Dorner T. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165:5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 20.Erickson LD, Lin LL, Duan B, Morel L, Noelle RJ. A genetic lesion that arrests plasma cell homing to the bone marrow. Proc Natl Acad Sci U S A. 2003;100:12905–12910. doi: 10.1073/pnas.2131686100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobi AM, Odendahl M, Reiter K, Bruns A, Burmester GR, Radbruch A, Valet G, Lipsky PE, Dorner T. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1332–1342. doi: 10.1002/art.10949. [DOI] [PubMed] [Google Scholar]
- 23.Jacobi AM, Mei H, Hoyer BF, Mumtaz IM, Thiele K, Radbruch A, Burmester GR, Hiepe F, Dorner T. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann Rheum Dis. 2010;69:305–308. doi: 10.1136/ard.2008.096495. [DOI] [PubMed] [Google Scholar]
- 24.Martin F, Chan AC. Pathogenic roles of B cells in human autoimmunity; insights from the clinic. Immunity. 2004;20:517–527. doi: 10.1016/s1074-7613(04)00112-8. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson RD, Lalor P, Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989;1:27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 27.Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, Muehlinghaus G, Szyska M, Radbruch A, Manz RA. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 28.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 29.Manz RA, Lohning M, Cassese G, Thiel A, Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- 30.Miller JJ, 3rd, Cole LJ. Resistance of long-lived lymphocytes and plasma cells in rat lymph nodes to treatment with prednisone, cyclophosphamide, 6-mercaptopurine, and actinomycin D. J Exp Med. 1967;126:109–125. doi: 10.1084/jem.126.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozono Y, Kotzin BL, Holers VM. Resting B cells from New Zealand Black mice demonstrate a defect in apoptosis induction following surface IgM ligation. J Immunol. 1996;156:4498–4503. [PubMed] [Google Scholar]
- 32.Jorgensen TN, Thurman J, Izui S, Falta MT, Metzger TE, Flannery SA, Kappler J, Marrack P, Kotzin BL. Genetic susceptibility to polyI:C-induced IFNalpha/beta-dependent accelerated disease in lupus-prone mice. Genes Immun. 2006;7:555–567. doi: 10.1038/sj.gene.6364329. [DOI] [PubMed] [Google Scholar]
- 33.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 34.William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol. 2005;174:6879–6887. doi: 10.4049/jimmunol.174.11.6879. [DOI] [PubMed] [Google Scholar]
- 35.McHeyzer-Williams MG, Ahmed R. B cell memory and the long-lived plasma cell. Curr Opin Immunol. 1999;11:172–179. doi: 10.1016/s0952-7915(99)80029-6. [DOI] [PubMed] [Google Scholar]
- 36.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 37.Marion TN, Bothwell AL, Briles DE, Janeway CA., Jr IgG anti-DNA autoantibodies within an individual autoimmune mouse are the products of clonal selection. J Immunol. 1989;142:4269–4274. [PubMed] [Google Scholar]
- 38.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 41.Sze DM, Toellner KM, Garcia de Vinuesa C, Taylor DR, MacLennan IC. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med. 2000;192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Connor BP, Cascalho M, Noelle RJ. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J Exp Med. 2002;195:737–745. doi: 10.1084/jem.20011626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169:4213–4221. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- 44.Roldan E, Brieva JA. Terminal differentiation of human bone marrow cells capable of spontaneous and high-rate immunoglobulin secretion: role of bone marrow stromal cells and interleukin 6. Eur J Immunol. 1991;21:2671–2677. doi: 10.1002/eji.1830211105. [DOI] [PubMed] [Google Scholar]
- 45.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, Noelle RJ. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 47.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 48.Austin HA, 3rd, Patel AD, Cadena CA, Boumpas DT, Balow JE. Ongoing immunologic activity after short courses of pulse cyclophosphamide in the NZB/W murine model of systemic lupus erythematosus. J Rheumatol. 1997;24:61–68. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.