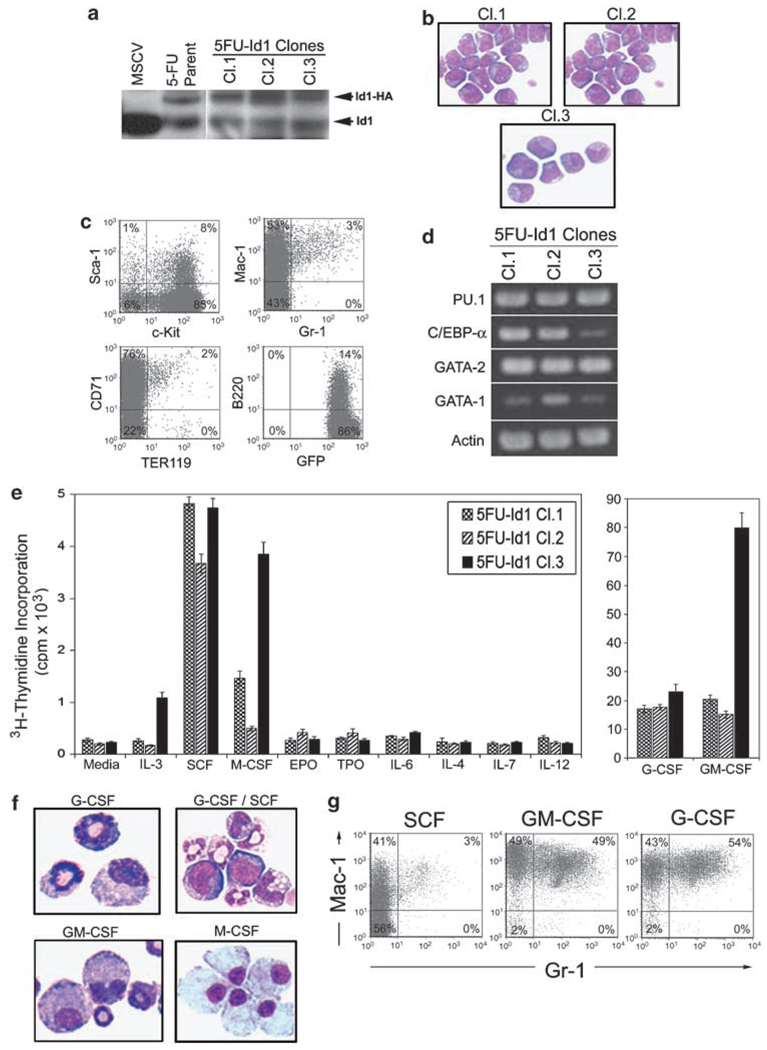
Figure 2.
Hematopoietic phenotype of cloned Id1 overexpressing bone marrow cells (BMC). BMC infected with retroviral vectors that express pMSCV-Id1-GFP were sorted and maintained in complete Iscove’s modified Dulbecco’s medium (cIMDM) with 10% fetal calf serum (FCS), P/S, 100 ng/ml mSCF, 100 ng/ml thrombopoietin (hTpo), 100 ng/ml hFlt-3L and 50 ng/ml hIL-6, then cloned by single cell culture. The stem cell factor (SCF)-dependent Id1 overexpressing green fluorescent protein (GFP) positive cells were maintained in cIMDM supplemented 100 ng/ml mSCF. (a) 5FU-Id1 immortalized BMC cloned cell lines (Cl.1, Cl.2, Cl3) and parent cells were examined for Id1 protein expression by western blot. BMC transfected with MSCV-GFP were used as control. Exogenous Id1 protein, tagged with HA, and endogenous Id1 are indicated, respectively. (b) Morphology of Wright–Giemsa stained 5FU-Id1 cloned cells; × 1000 magnification. (c) Cloned cells were analysed by flow cytometry for the expression of hematopoietic developmental surface markers. Isotype control antibodies were used as negative controls to exclude nonspecific background staining. Percentages of positive cells are indicated in each quadrant. (d) Reverse transcriptase (RT)-PCR products for hematopoietic transcriptional regulators (PU.1, C/EBPα, GATA-2, GATA-1) in 5FU-Id1-cloned cell lines were shown. (e) Proliferation of 5FU-Id1 cell lines indicated by 3H-thymidine incorporation in the presence of the indicated cytokine. Bars indicate average 3H-thymidine incorporation in c.p.m. × 103 over 16 h as measured from triplicate wells ± s.e.m. (f, g) For induction of myeloid differentiation, cloned cells were cultured with hG-CSF (100 ng/ml), mGM-CSF (100 ng/ml) or hM-CSF (100 ng/ml) for 5 days. Morphology and flow cytometry analysis of myeloid cell surface markers (Gr-1, Mac-1) on 5FU-Id1 Cl.2 cells after culture for 5 days in the indicated cyto were shown (Wright–Giemsa, × 1000 magnification).