Table 1.
TG2 inhibitory activity of enantiopure azido- and alkynyl-DHI compounds. Compounds 17 and 18 have a stereochemically altered DHI moiety, and are therefore considerably less potent than their diastereomeric analogs, 11 and 12, respectively.
| Cmpd | K I(μM) | kinh (min−1) | kinh/KI(mM−1 min−1) | Cmpd | K I(μM) | kinh (min−1) | kinh/KI(mM−1 min−1) |
|---|---|---|---|---|---|---|---|
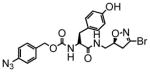 3 |
280 | 1.63 | 5.8 |
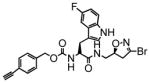 11 |
7.0 | 0.13 | 19 |
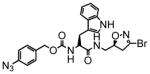 4 |
15 | 0.23 | 15 |
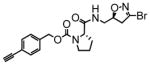 12 |
1.8 | 0.052 | 30 |
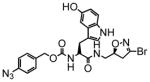 5 |
100 | 0.82 | 8.0 |
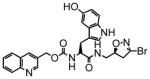 13 |
41 | 0.51 | 12 |
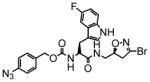 6 |
19 | 0.31 | 16 |
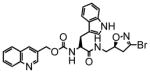 14 |
47 | 0.53 | 11 |
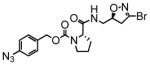 7 |
9.2 | 0.12 | 13 |
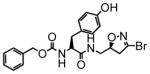 15 |
22 | 0.13 | 6.1 |
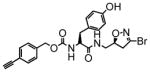 8 |
4.2 | 0.016 | 4.0 |
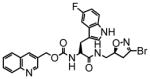 16 |
19 | 0.73 | 38 |
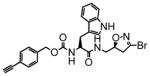 9 |
13 | 0.15 | 11 |
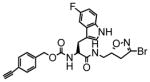 17 |
51 | 0.21 | 4.2 |
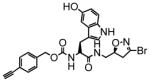 10 |
12 | 0.14 | 8.0 |
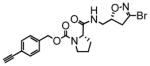 18 |
38 | 0.27 | 7.2 |
