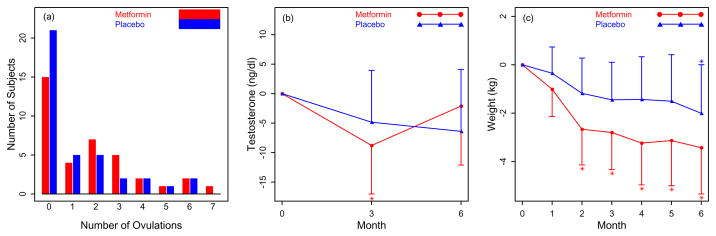
Figure 2.
Primary Outcome A Frequency of the number of ovulations by Urinary PdG per treatment arm. There was no difference between groups. This model adjusted for the covariates of prior metformin use, center, baseline age, and baseline body mass index in addition to use of the logarithm of the number of days on the trial as on offset to help account for subject dropout. Secondary Outcomes B. Change in testosterone levels at baseline, 3 mos and study completion at 6 mos per treatment arm. C. Change in weight (kg) by month per treatment arm. * P < 0. 05 for the change from baseline within the metformin group.