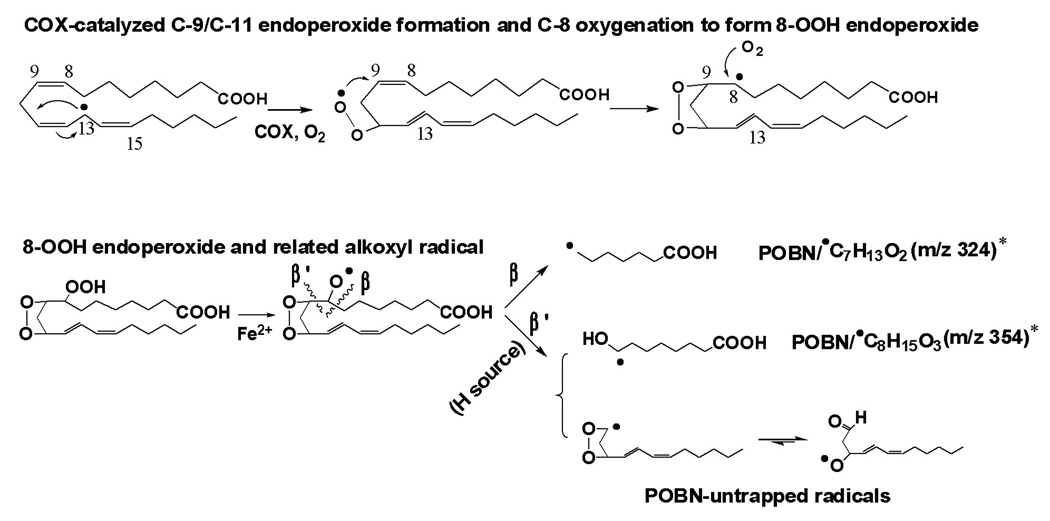
Scheme 2.
Proposed mechanism of COX-DGLA peroxidation to form DGLA-derived free radicals exclusively via C-8 oxygenation.
Note that in DGLA peroxidation, C-8 oxygenation (addition of the second O2), after formation of the C-13 radical and C-9/C-11 endoperoxide bridge (addition of the first O2), corresponds to the formation of carboxyl end carbon-centered radicals and oxygen-centered radicals. The H source needed for β’-scission in this pathway could be hydrophilic Ser-530 and Gly-526 of COX channel residues as well as H2O [29–30]. The proposed oxygen-centered radicals can be further decomposed to the other products, e.g. C-9 to C-11 elimination to form malonaldehyde.
* In the case of peroxidation of DGLA-methyl ester, m/z 338 and m/z 368 of the POBN adduct of •C8H13O2 (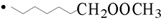 ) and •C9H17O3 (
) and •C9H17O3 ( ) are observed instead of m/z 324 and m/z 354.
) are observed instead of m/z 324 and m/z 354.