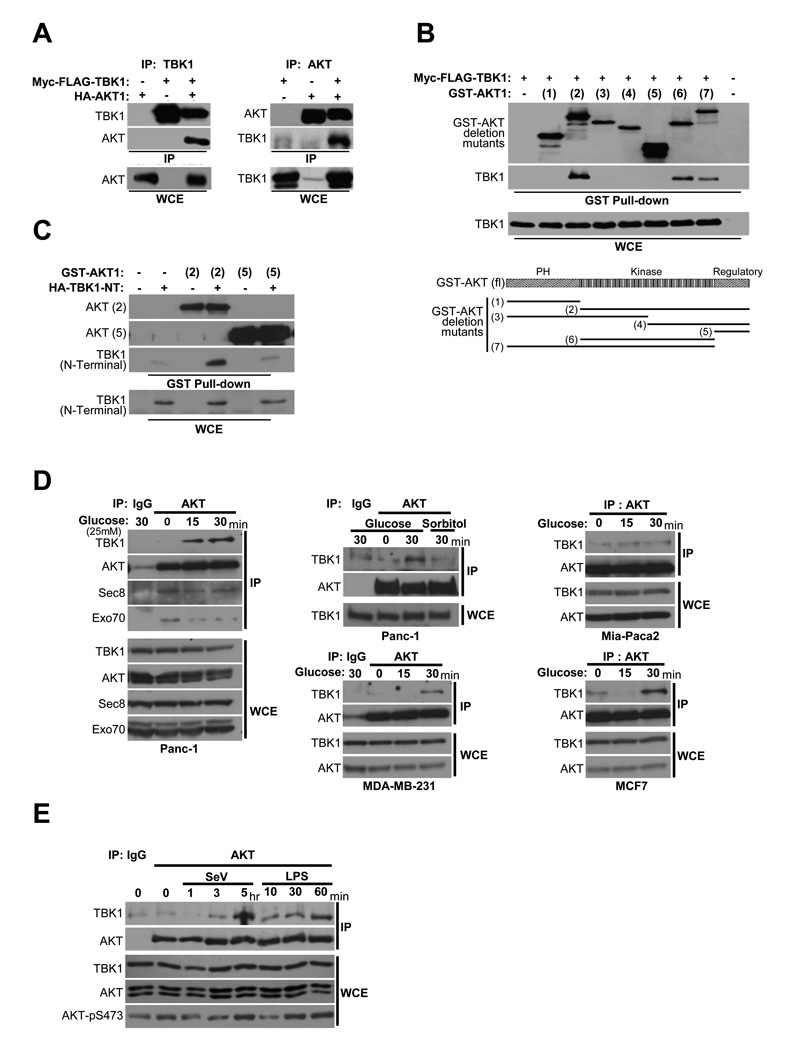
Figure 3. TBK1/AKT complex formation is stimulus-specific.
(A) HEK293T cells were transfected as indicated. Reciprocal co-expression/co-immunoprecipitations are shown. (Molecular size: as previously described)
(B) GST-AKT expression constructs encoding a panel of truncation variants were coexpressed with Myc-FLAG-TBK1 in HEK293T cells. Glutathione-mediated affinity isolation of the AKT variants (GST Pull-down) was used to define a minimally sufficient TBK1 interaction domain as indicated. Whole cell extracts (WCE) are shown as controls for TBK1 expression.
(C) HA-tagged TBK1 amino-terminal fragment (1–242) that encompasses the catalytic domain [TBK1 (N-terminal)] was coexpressed with either GST-AKT expression constructs [AKT(2)] or [AKT(5)]. Affinity isolation of AKT was probed for co-isolation of N-terminal TBK1as in (B).
(D) Panc-1, MDA-MB-231, Mia-Paca2, and MCF7 cells were deprived of glucose for 2 hr followed by incubation with 25 mM glucose or sorbitol as indicated. Endogenous AKT was immunoprecipitated from extracts taken at the indicated time points. Immunoprecipitates were assayed for coprecipitation of the indicated proteins. Normal mouse IgG was used as a control for specificity (IgG lanes). (Molecular size: as described)
(E) H1993 cells were either exposed to Sendai virus (SeV, 100 HA/ml) or treated with LPS (1 µg/ml), and harvested at the indicated time intervals. Co-immunoprecipitation and immunoblot were performed as in (D). Normal mouse IgG was used as a control for specificity (IgG lane). (Molecular size: as described)